��Ŀ����
��������������� (����)
| A����a L 0.1 mol/L ��CH3COOH��Һ��b L 0.1 mol/L�� KOH��Һ��ϣ������� Һ��һ�����ڣ�c (K+)+ c (H+) =" c" (CH3COO��) + c (OH��) |
| B����ˮ�У�c(Cl��)��c(H+)��c(OH��)��c(ClO��) |
| C����0.1 mol/L ��NaHCO3��Һ��0.3 mol/L ��Ba(OH)2��Һ�������ϣ������� Һ��һ�����ڣ�c (OH��) >c (Ba2+)>c (Na+)> c (H��) |
| D�������£���pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶���ͬ |
B
�������������A������غ�,��ȷ��B��c(ClO��) ��c(OH��)��OH����ˮ�����������С������C��HCO3��+OH-= CO32-+ H2O��Ba2++ CO32-=" Ba" CO3��, c(OH��)="0.5" ,c (Ba2+)="0.2," c (Na+)="0.1," c (H��)��ˮ��������ģ���С������c (OH��) >c (Ba2+)>c (Na+)> c (H��)����ȷ��D��CH3COOH��Һc (H+)����pH=11��NaOH��Һ��c(OH��)��Ϊ10-3��Kw =10-14 �������H+ ��OH�� ��ȣ���ˮ��������ģ���ȷ��
���㣺������Һ�е�����Ũ�ȱȽϵ����֪ʶ��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����������ȷ����
| A����pH��2�������pH��2�Ĵ���������ϣ���Ϻ���Һ��pH��2 |
| B��HA-��HB-���ѵ��룬��NaHA��Һ��pHһ����NaHB��Һ�Ĵ� |
| C���ڱ��Ͱ�ˮ�м���ͬŨ�ȵİ�ˮ��ƽ�������ƶ� |
| D��ij�¶��£�Ba(OH)2��Һ�У�Kw��10-12��pH��8�ĸ���Һ�м�������pH��4�����ᣬ�����Һ��pH��7 |
�����£�Ũ�Ⱦ�Ϊ0.1000 mol ? L ��1������һԪ��HX��HY��HZ���ֱ���0. 1000 mol ? L-1��NaOH��Һ���еζ����ζ�������ͼ��ʾ�������й�������ȷ����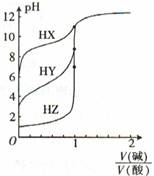
| A��NaOH��Һ��������ķ�Ӧ�������ȷ�Ӧ |
| B������ǿ����HX>HY>HZ |
| C���ζ���HY���÷�̪��ָʾ�� |
| D���ζ������ᶼ���ü�����ָʾ�� |
ij�¶�ʱ��BaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ����( )
| A������Na2SO4��ʹ��Һ��a��䵽b�� |
| B��ͨ����������ʹ��Һ��d��䵽c�� |
| C��d����BaSO4�������� |
| D��a���Ӧ��Ksp����c���Ӧ��Ksp |
�����й�˵����ȷ����
| A��Hg(l) + H2SO4(aq) = HgSO4(aq) + H2(g)�����²����Է����У�˵����H��0 |
| B���ö��Ե缫���1L1mol/L��CuSO4��Һ������������3.2 gͭʱ������0.05 molCu(OH)2����ɽ���Һ�ָ���ԭŨ�� |
| C����֪25��ʱ��Ksp(AgCl)=1.8��10��10��Ksp(Ag2CrO4)=2.0��10��12������AgCl���ܽ�ȴ���Ag2CrO4���ܽ�� |
| D��25��ʱ����0.1 mol/L CH3COOH��Һ�м�������CH3COONa���壬����Һ��ˮ�ĵ���̶Ƚ�������Kw���� |
���ڵ������Һ����˵���в���ȷ����
A����Ũ�Ⱦ�Ϊ �����Һ����μ��백ˮ����������ɫ �����Һ����μ��백ˮ����������ɫ �������� �������� |
| B���õ�Ũ�ȵ�NaOH��Һ�к͵����pH=2��pH=3�Ĵ��ᣬ�����ĵ�NaOH��Һ�����ǰ���Ǻ��ߵ�10�� |
C���� ��Һ�������ϣ�������Ũ�ȵ�˳��Ϊ�� ��Һ�������ϣ�������Ũ�ȵ�˳��Ϊ�� |
D��ij�¶��´�ˮ�� ������¶���0.1mol/L�������pH=1 ������¶���0.1mol/L�������pH=1 |
���й��ڵ������Һ��������ȷ����
| A�������£�Na2CO3��Һ��pH>7 |
| B��0.1mol/L Na2CO3��Һ��35��ʱ���Ա�25��ǿ��˵����ˮ�ⷴӦ���Ƿ��ȷ�Ӧ |
| C�������£�pH=7��NH4Cl�백ˮ�Ļ����Һ�и�����Ũ�ȵĹ�ϵΪ�� c(Cl-)=c(NH4+)>c(H+)=c(OHһ) |
| D�������£��к�pH���������ͬ������ʹ��ᣬ����NaOH�����ʵ�����ͬ |
���й�ϵ��ȷ����
| A���������ʵ�����H2CO3��KHCO3����ˮ��ɵ���Һ�У� 2c��K+��= c(H2CO3)+c��HCO3-�� |
B����CH3COONa��Һ�����������NaOH�����õ��Ļ����Һ�У� |
C����0��10 mol��L-1 NH4HSO4��Һ�еμ�������NaOH��Һ����Һ�����ԣ���������Һ�У� |
| D����NaCN��Һ�еμ�������ϡ����ʹ��Һ�����ԣ���������Һ�У� |

���ڳ�����0.1mol��L��1 NaHCO3��Һ��������������ȷ���ǣ� ��
| A��c (Na��)="c" (HCO3��) + c (CO32��) + c (H2CO3) |
| B���¶����ߣ���Һ��pH���ߣ�����Һ�е�c��H������c��OH�����˻����� |
| C����������Ũ�ȵ�CH3COOH��Һ��Ӧ����Һ��c��Na������c��CH3COO���� |
| D������������NaOH���壬��Һ��pH��С |