��Ŀ����
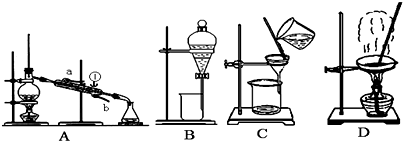
��14�֣��������ķ������ᴿ�ڻ�ѧʵ����ռ����Ҫ��λ�á���ͼ��ʾ�ӹ��������з���X�ķ�������ش��й����⡣
��1��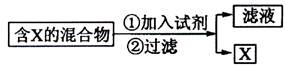
����������ͼʾ��ij������ĩ(����Au��Ag��Cu)�з���Au��������Լ��� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϊ�ᴿijFe2O3��Ʒ(��Ҫ������SiO2��A12O3)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽��(ע�����ʺͲ���) ��
����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ��
��1���ù��յ��м���̻ᷢ����Ӧ�� ����Ӧ����������______________����������Ϊ__________
����Ӧ����������______________����������Ϊ__________
��2���ھ���ͭ�Ĺ����У����Һ��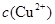 ���½���
���½���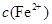 ��
��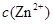 �������ӣ������趨ʱ��ȥ���е�
�������ӣ������趨ʱ��ȥ���е�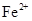 ��
��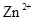 ���±�Ϊ�������ʵ��ܶȻ���
���±�Ϊ�������ʵ��ܶȻ���
| ���� |  | 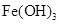 | 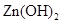 |  |
�ܶȻ�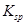 | 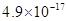 | 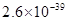 | 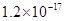 | 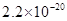 |
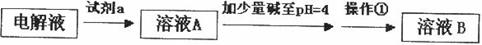
���Լ�a��__________����Ŀ����____________________________________�������ܶȻ��÷����ܹ���ȥ�����ʽ�����������____________��д����������ʽ��������ӵIJ���������____________________________________________________________________________.
����ͬѧ�ڲ�������ʱ���֣�����ҵԭ���Ȼ���к������Ȼ�����ʹ������ˮ���ټ��백ˮ
����pH��7��8����ʹ
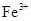 ����
����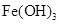 ����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ
����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����ҲӦ�ý���ҺpH����7��8������Ϊ��ͬѧ�Ľ����Ƿ���ȷ?________(��ǡ���)��
������________________________________________________________________________��
����1��Ũ���ᣬAg+2HNO3(Ũ) = AgNO3 + NO2 ��+ H2O��
Cu + 4 HNO3(Ũ) = Cu(NO3)2 + 2NO2 ��+2 H2O
��2��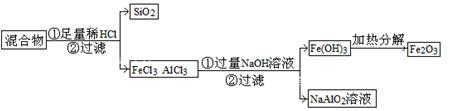
����1��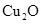 ,
,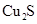 SO2��2����
SO2��2����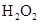 ��
��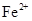 ������
������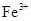
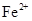 �ȼ���KSCN��Һ������Һ����죬�ٵ��뼸����ˮ�������ᣩ������Һ��ɺ�ɫ��˵�����и����ӡ��ڷ� �����ܶȻ�������ҺpHΪ7~8ʱ����ҺA��
�ȼ���KSCN��Һ������Һ����죬�ٵ��뼸����ˮ�������ᣩ������Һ��ɺ�ɫ��˵�����и����ӡ��ڷ� �����ܶȻ�������ҺpHΪ7~8ʱ����ҺA��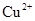 ͬʱ���ɳ���������ȥ��
ͬʱ���ɳ���������ȥ��
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��������ķ������ᴿ�ڻ�ѧʵ����ռ����Ҫ��λ�á���ͼ��ʾ�ӹ��������з���X�ķ�������ش��й����⡣
![]() ��1��
��1��
����������ͼʾ��ij������ĩ(����Au��Ag��Cu)�з���Au��������Լ��� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
![]() ��2��Ϊ�ᴿijFe2O3��Ʒ(��Ҫ������SiO2��A12O3)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽��(ע�����ʺͲ���) ��
��2��Ϊ�ᴿijFe2O3��Ʒ(��Ҫ������SiO2��A12O3)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽��(ע�����ʺͲ���) ��
����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ��
��1���ù��յ��м���̻ᷢ����Ӧ�� ����Ӧ����������______________����������Ϊ__________
����Ӧ����������______________����������Ϊ__________
��2���ھ���ͭ�Ĺ����У����Һ��![]() ���½���
���½���![]() ��
��![]() �������ӣ������趨ʱ��ȥ���е�
�������ӣ������趨ʱ��ȥ���е�![]() ��
��![]() ���±�Ϊ�������ʵ��ܶȻ���
���±�Ϊ�������ʵ��ܶȻ���
| ���� |
|
|
|
|
| �ܶȻ� |
|
|
|
|
��ͬѧ��������³��ӷ�����
![]()
���Լ�a��__________����Ŀ����____________________________________�������ܶȻ��÷����ܹ���ȥ�����ʽ�����������____________��д����������ʽ��������ӵIJ���������____________________________________________________________________________.
����ͬѧ�ڲ�������ʱ���֣�����ҵԭ���Ȼ���к������Ȼ�����ʹ������ˮ���ټ��백ˮ
����pH��7��8����ʹ![]() ����
����![]() ����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ
����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ
Ӧ�ý���ҺpH����7��8������Ϊ��ͬѧ�Ľ����Ƿ���ȷ?________(��ǡ���)��
������________________________________________________________________________��
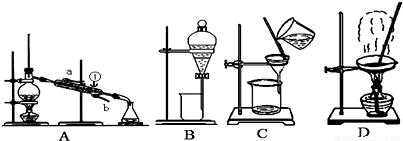
��1�����Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ��______�������װ��ͼ����ĸ����ͬ������ȥ����ˮ�е�Cl-�����ʣ�ѡ��װ��______��
��2���ӵ�ˮ�з����I2��ѡ��װ��______���÷��뷽��������Ϊ______��
��3��װ��A�Тٵ�������______������ˮ�ķ�����______��
��4��װ��B�ڷ�ҺʱΪʹҺ��˳���µΣ�Ӧ���еľ��������______���������ѡ�����ĸ��
A��Ӧ���Ƚ���Һ©���ϵĻ������º��ٴ������ų�Һ��
B������
C��ʹ��Һ©���ϵĻ����ϵİ��۶���Һ©���ϵ�С���ٴ������ų�Һ�壮
��ijͬѧ�����в�������250mL 0.2mol?L-1NaCl��Һ����ش��й����⣮
| ʵ�鲽�� | �й����� |
| �ټ�������NaCl������ | |
| �ڳ���NaCl���� | ��������ƽ��Ҫ����NaCl������Ϊ______g�� |
| �۽�NaCl����100mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ��______�� |
| �ܽ��ձ�����Һת����250mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ��______�� |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ���̶���1-2���״�Ӧ��β����� ______�� |
��2��ȡ����NaCl��Һ10mL��ˮϡ�͵�100mL��ϡ�ͺ���Һ��Na+�����ʵ���Ũ����______��
��3�������Тݲ�����ʱ������ˮ�����̶��ߣ�����______��
��4������NaCl��Һʱ�����в��������ʹ���ƫ�ߵ���______��
A����Һǰ������ƿ������������ˮ
B���ܽ����ʱ��Һ��ɽ�
C������ʱ��������ƿƿ���̶���
D�����ݺ�������ƿҡ�Ⱦ�ƽ�ž��ã�Һ����ڿ̶��ߣ��ټ�ˮ���ݣ�