��Ŀ����
Fe��OH��2��һ�ְ�ɫ�������ڿ����л��ں���O2��ˮ�����ױ���������ɫ�����Կ������ġ�����Ŀ����Ϊ�˽��������⣬�����ѧ�ҶԴ�ʵ�������о��Ľ�����������ͼ��һ���Ĵ����ԡ�
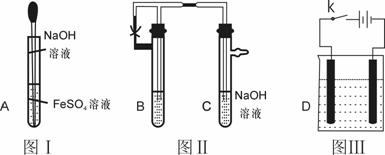
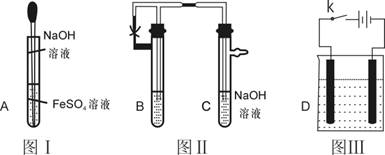
��1�����ճ���ʵ���Ҫ���ý�ͷ�ιܵμ���Һʱ ����ܡ����ܡ��������Թ��У�ԭ���� ��ͼI�У�����ͷ�� ������Һ���µ�Ŀ���� ��
��2����ͼ���У�Ҫ�۲쵽Fe��OH��2�����ɣ�B���е��Լ�������
ʵ������IJ����ǣ���Ӧ�Լ��Ѽ��룩
��
��
����������ٺ͢ڵߵ����ܷ�Fe��OH��2��ɫ��������˵�����ɣ�
��3������ͼ��װ��ʵ�飬��������Ӧѡ�� ,������ʯī��������Ӧ ��������Ӧ�� ����ʵ����һ���ԵIJ��㣬�����ڳ�������ؿ���Fe��OH��2��ɫ���������ɣ���ָ�����跨�˷��� ��

��1�����ܣ�ҩƷ�ᱻ��Ⱦ����ֹ�������������ҩƷ����������
��2��ϡ���� ��1��ֹˮ�д�C����Һ�д�������2�ر�ֹˮ��
���ܣ���
��3��Fe,Fe-2e��=Fe2+,2H++2e��=H2��,��Һ������Ӵ���������Fe��OH��2���ʣ���������Һ�ϲ�ӸDZ���Һ���籽��


 [Fe2��OH��4]2++2H+
[Fe2��OH��4]2++2H+