��Ŀ����
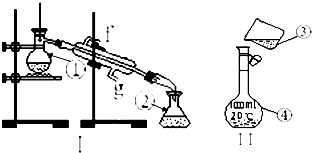 ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã���1��д�����б�Ŷ�Ӧ����������
�٣�
������ƿ
������ƿ
���ڣ�
������
������
���ܣ�
����ƿ
����ƿ
����2�������١����У�ʹ��ʱ�������Ƿ�©ˮ����
��
��
��������ţ���3��������װ�â�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������
�ƾ���
�ƾ���
��������������������е�ʵ�����������Ϊ����
����
��ʵ�������ͨ����ȴˮ�ķ�����g��f
g��f
���g��f������f��g��������4��������250mL 0.2mol/L NaCl��Һ��װ�â���ijͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����
δ�ò�����������δ����250mL����ƿ
δ�ò�����������δ����250mL����ƿ
�����ľ���и������Σ���Ҫ�ɷ���̼��أ������������Ȼ��صȣ��ִӲ�ľ������ȡ���Σ�����ʵ��������е� CO32-��SO42- �� Cl-��
��1���Ӳ�ľ������ȡ���ε�ʵ�鲽���ǣ��ٳ��������ܽ���۹��ˡ��ܣ��ٹ��ˣ�������Һ����������ȴ�ᾧ
��2����������ƽ��ָ�����ϵģ�������Ʒʱ����ָ��ƫ���ұߣ����ʾ
B
B
����ѡ��ı�ţ���A�������أ���Ʒ�� B�������ᣬ������
C�������أ������� D�������ᣬ��Ʒ��
��3���ڽ��еڢ۲�����ʱ����ʱ����Ҫ�ظ����У���������
��Һ�л��в���������
��Һ�л��в���������
����4�����Ƶõ�������������Թܣ���������ˮ�ܽ⣬���֤����Һ����SO42-��
ȡ������Һ���Թ��У�����ϡ������ټ��Ȼ�����Һ���а�ɫ����������˵������SO42-����Ӧ����ʽΪBa2++SO42-�TBaSO4��
ȡ������Һ���Թ��У�����ϡ������ټ��Ȼ�����Һ���а�ɫ����������˵������SO42-����Ӧ����ʽΪBa2++SO42-�TBaSO4��
����������1�����������Ľṹ�����ж����������ƣ�
��2������ƿ��ʹ��ǰһ��Ҫ��©��
��3���������Ȼ�̼�;ƾ��Ļ����Ĺ��̱����þƾ��Ƽ��ȣ�ʵ����������̣��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
��4����������һ�����ʵ���Ũ�ȵ���Һ�ķ����Ͳ�����Ѱ��װ���еĴ���
��2��������ƽƽ���ԭ��������Ʒ�������̣�����������̣���ָ��ƫ���ұߣ����ұ��أ�
��3����Һ�л��в��������ʣ�
��4������Ba2+��SO42-��Ӧ���ɲ�����ˮ��������ᱵ��ɫ��������SO42-�������Լ�Ϊ���ᡢ�Ȼ�����Һ��
��2������ƿ��ʹ��ǰһ��Ҫ��©��
��3���������Ȼ�̼�;ƾ��Ļ����Ĺ��̱����þƾ��Ƽ��ȣ�ʵ����������̣��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
��4����������һ�����ʵ���Ũ�ȵ���Һ�ķ����Ͳ�����Ѱ��װ���еĴ���
��2��������ƽƽ���ԭ��������Ʒ�������̣�����������̣���ָ��ƫ���ұߣ����ұ��أ�
��3����Һ�л��в��������ʣ�
��4������Ba2+��SO42-��Ӧ���ɲ�����ˮ��������ᱵ��ɫ��������SO42-�������Լ�Ϊ���ᡢ�Ȼ�����Һ��
����⣺��1������װ���е���Ҫ������������ƿ�������ܡ�ţ�ǹܡ���ƿ���ƾ��ƣ�aΪ������ƿ��bΪ�����ܣ�cΪ����ƿ��Ϊ������Һ����Ҫ������
�ʴ�Ϊ��������ƿ�������ܣ�g��f��
��2��������ƿ��ʹ��ǰһ��Ҫ��©��������鷽����������ƿ�м�������ˮ������ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ�������۲�ƿ����Χ�Ƿ���ˮ©�����粻©ˮ����ƿ������ƿ����ת180�㣬�ظ�����������
�ʴ�Ϊ���ܣ�
��3���������Ȼ�̼�;ƾ��Ļ�����ʵ����������̣������þƾ��ƣ��¶ȼ�Ӧλ��������ƿ֧�ܿڣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ����� g��f��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ���ʴ�Ϊ��δ�ò�����������δ����250mL����ƿ��
��2����������ƽ������Ʒʱ����ָ��ƫ���ұߣ����ʾ�����ᡢ�����أ�
�ʴ�Ϊ��B��
��3����Һ�л��в��������ʣ������¹��ˣ�
�ʴ�Ϊ����Һ�л��в��������ʣ�
��4������SO42-�ķ���Ϊ��ȡ������Һ���Թ��У�����ϡ������ټ��Ȼ�����Һ���а�ɫ����������˵������SO42-����Ӧ���ӷ���ʽΪ��Ba2++SO42-�TBaSO4����
�ʴ�Ϊ��ȡ������Һ���Թ��У�����ϡ������ټ��Ȼ�����Һ���а�ɫ����������˵������SO42-��Ӧ���ӷ���ʽΪ��Ba2++SO42-�TBaSO4����
�ʴ�Ϊ��������ƿ�������ܣ�g��f��
��2��������ƿ��ʹ��ǰһ��Ҫ��©��������鷽����������ƿ�м�������ˮ������ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ�������۲�ƿ����Χ�Ƿ���ˮ©�����粻©ˮ����ƿ������ƿ����ת180�㣬�ظ�����������
�ʴ�Ϊ���ܣ�
��3���������Ȼ�̼�;ƾ��Ļ�����ʵ����������̣������þƾ��ƣ��¶ȼ�Ӧλ��������ƿ֧�ܿڣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ����� g��f��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ���ʴ�Ϊ��δ�ò�����������δ����250mL����ƿ��
��2����������ƽ������Ʒʱ����ָ��ƫ���ұߣ����ʾ�����ᡢ�����أ�
�ʴ�Ϊ��B��
��3����Һ�л��в��������ʣ������¹��ˣ�
�ʴ�Ϊ����Һ�л��в��������ʣ�
��4������SO42-�ķ���Ϊ��ȡ������Һ���Թ��У�����ϡ������ټ��Ȼ�����Һ���а�ɫ����������˵������SO42-����Ӧ���ӷ���ʽΪ��Ba2++SO42-�TBaSO4����
�ʴ�Ϊ��ȡ������Һ���Թ��У�����ϡ������ټ��Ȼ�����Һ���а�ɫ����������˵������SO42-��Ӧ���ӷ���ʽΪ��Ba2++SO42-�TBaSO4����
���������⿼��ѧ����ѧʵ��Ļ�������֪ʶ�����Ը�����ѧ֪ʶ���ش𣬽ϼ�ѧϰ��ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
 �������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
 �������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�