��Ŀ����
����ѡһ����ѡ��2����ѧ�뼼����
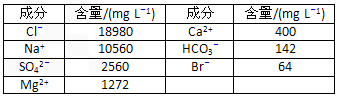
�����к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�顣
�����к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�顣
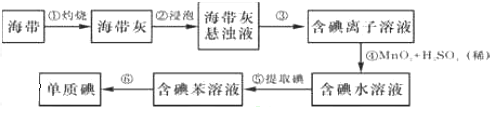
����д���пհף�
��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������____________��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
A.�ձ� B.���� C.������ D.������ E.�ƾ��� F.������
��2������۵�ʵ�����������____________�������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������___________��
��3������ܷ�Ӧ�����ӷ���ʽ�� ______________��
��4��������У�ijѧ��ѡ���ñ�����ȡ���������_____________ ��
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�_____________��
��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������____________��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
A.�ձ� B.���� C.������ D.������ E.�ƾ��� F.������
��2������۵�ʵ�����������____________�������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������___________��
��3������ܷ�Ӧ�����ӷ���ʽ�� ______________��
��4��������У�ijѧ��ѡ���ñ�����ȡ���������_____________ ��
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�_____________��
��1��BDE
��2������ ������
��3��2I��+MnO2+4H+=Mn2+ +I2+2H2O
��4������ˮ�������ܣ����ڱ��е��ܽ�ȱ���ˮ�д�
��5��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩
��2������ ������
��3��2I��+MnO2+4H+=Mn2+ +I2+2H2O
��4������ˮ�������ܣ����ڱ��е��ܽ�ȱ���ˮ�д�
��5��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩

��ϰ��ϵ�д�
�����Ŀ