��Ŀ����
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������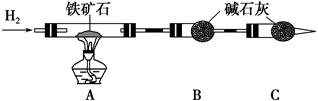
��1������ͼ��װ��������______________________________________________��
��2����8.0 g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
��3������˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
��4����ַ�Ӧ�����ƾ��ƣ�________________________________________��
��5����÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
��.����ʯ�к������IJⶨ���������¡�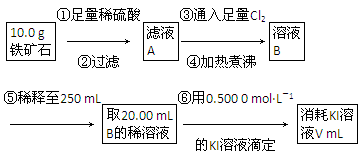
��1�����������������___________________________________________��
��2����������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
��3�������йز���IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
��4�����ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�ɢ���������������ʯ������������Ļ�ѧʽΪ________��
��.��1�����װ�õ������ԡ���3����װ��C���ڴ������鴿��4���ٳ���ͨ��������Ӳ�ʲ�������ȫ��ȴ����5��25.0%
��.��1��������Һ���ܽ�Ĺ���Cl2����2������������3��be����4��70.0%
��.Fe4O5
����

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д����Ρ��������ڹ�ũҵ�����������ˮ�����ȷ������ż���㷺��Ӧ�ã�
��һ������м�����ᷴӦ�Ʊ�FeSO4
��֪4Fe2++O2+4H+= 4Fe3++2H2O��FeSO4��ˮ�е��ܽ�ȼ�ͼ��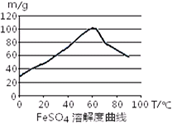
��1�����ȣ�����м����̼������Һ����г����ۣ������Һ�壬��ˮϴ����м���˲����У������Һ��ķ���ͨ�����ù��ˣ�ʹ�õIJ�����____________����д�������ƣ���
��2������������м�м������������ᣬ��һ���¶���ʹ�䷴Ӧ�����ٲ������壬���ȹ��ˣ���FeSO4��Һ���˴�������Ũ��Ӧѡ��
| A��Ũ���� | B��10moL/L���� | C��3moL/L���� | D������Ũ����� |
��3���������ڿ������ױ����������γɸ��ο��ȶ����ڡ��硰Ħ���Ρ��������������[��NH4��2SO4?FeSO4?6H2O]��������FeSO4��Һ�м�������ϡH2SO4��Һ���ټ��뱥�ͣ�NH4��2SO4��Һ����������Ũ������ȴ�ᾧ�����˵�һϵ�в��������á�������ϡ����������� ����
�������ú�������Al�ķ���м�Ʊ�Fe2(SO4)3��������̼��й��������£�
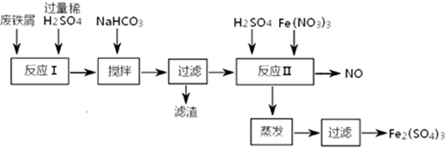
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2��3 | 7��5 | 3��4 |
| ��ȫ���� | 3��2 | 9��7 | 4��4 |
��4����NaHCO3ʱ�����ҺpHֵӦ������_______________________��
��5����Ӧ���з�Ӧ�����ӷ���ʽ�ǣ�___________________��
��6��ʵ�������У�����Ӧ�������NO���һ������X����Ϻ�����ͨ�뷴Ӧ���У�����Ƶ�Ŀ����________������X��NO��ȵı�����_____________��
ij�о�С��������ͼװ��̽���¶ȶ�CO��ԭFe2O3��Ӱ��(�̶�װ����)��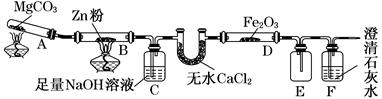
(1)MgCO3�ķֽ����Ϊ________��
(2)װ��C��������_____________________________________________________��
����β���ķ���Ϊ_______________________________________________________��
(3)���о�С���Ϊ���飬����ͼװ�ý��жԱ�ʵ�飬�����þƾ��ơ������þƾ���ƶ�װ��D���ȣ���Ӧ�����Ϊ��ɫ��ĩ(������)������ֱ��ò����������ʵ�飺
(��֪��Fe2����K3[Fe(CN)6]��Ӧ������ɫ����)��
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ����ϡ���� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�K3[Fe(CN)6]��Һ | ��ɫ���� | ��ɫ���� |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
������õ��ĺ�ɫ��ĩ��________��
�ڼ��鲽��1�з�Ӧ�����ӷ���ʽΪ__________________________________________��
�����鲽��4�У���Һ����ԭ��Ϊ_______________________________________��
��Һ��ɫ���ܵ�ԭ������֤����Ϊ______________________________________
�ܴ�ʵ�鰲ȫ���ǣ���ͼװ�û��ɲ�ȡ�ĸĽ���ʩ��__________________________
ij��ѧС����ѧϰԪ�������ɺ�,�Խ̲���Fe2+����ΪFe3+��ʵ���һ��˼��,���������:Cl2�ܽ�Fe2+����ΪFe3+,��ôBr2��I2�ܷ�Fe2+����ΪFe3+?
����һ:�����Ʋ�
����ͬѧ��ΪBr2��I2�����ܽ�Fe2+����ΪFe3+,����˼������������ ��
����ͬѧ��ΪBr2��I2�����ܽ�Fe2+����ΪFe3+,����ͬѧ��ΪBr2�ܽ�Fe2+����ΪFe3+��I2���ܡ�����˼���������Ǵ��ϵ���±�ص��������Լ�����
���ڶ�:���ʵ�������֤
�ڴ��Թ��м���������,����10 mLϡ����,���Թ�,��ַ�Ӧ��,������ʣ��,ȡ�ϲ���Һ��������ʵ�顣
ʵ��1:
| �Թ� | ���� | ���� |
| �� | �����Թ��м���2 mL FeCl2��Һ,�ٵμ���������ɫ����ˮ,���Թ� | ��ҺΪ��ɫ |
| �� | �����Թ��м���2 mL FeCl2��Һ,�ٵμ������ػ�ɫ�ĵ�ˮ,���Թ� | ��ҺΪ��ɫ |
(1)ͬѧ����Ϊ��������˵����ˮ�ܽ�Fe2+����,���ӷ���ʽΪ�� ��
ͬѧ����ΪӦ�ò���ʵ��,���ܵó�ͬѧ�Ľ��ۡ��������ͬѧ�����ʵ��:
ʵ��2:
| ���� | Ӧ�ù۲쵽������ |
| | |
(2)��С��ͬѧ�Ԣ�����Һ�ʻ�ɫ��ԭ��չ��������:
����1:��ˮ��FeCl2��Һ����Ӧ,��ɫ�ǵ�ˮϡ�ͺ����ɫ��
����2:�� ��
ʵ��3:����ʵ����ȷ�����ܵ�ԭ��
| ���� | ���� |
| ���Թܢ�������Һ�м�������0.5 mL CCl4,�����,����һ��ʱ���ȡ���ϲ���Һ,�μ�KSCN��Һ | ���ú�,�ϲ���Һ������ɫ,�²���ҺΪ��ɫ;�ϲ���Һ�μ�KSCN��Һ��,����dz��ɫ |
ͬѧ����Ϊ��ʵ���������˵���ǡ�����2������,ͬѧ����Ϊ���Ͻ�,���������ʵ��4:
ʵ��4:
| ���� | ���� |
| ����һ֧�Թ��м���2 mL FeCl2��Һ,�μ�0.5 mL��ˮ��,�ټ���0.5 mL��������,�����,����һ��ʱ���ȡ���²���Һ,�μ�KSCN��Һ | ���ú�,�ϲ�ҺΪ��ɫ,�²�Һ������ɫ;�²���Һ�μ�KSCN��Һ��,û�г���dz��ɫ |
����Ϊʵ��4��Ƶ���ҪĿ���� ����
ͬѧ������ʵ��4����ó�����:�ڱ���ʵ��������,��ˮ��FeCl2��Һ��Ӧ�ij̶Ⱥ�С��
(3)Cl2��Br2��I2����Fe2+����������,��ԭ�ӽṹ����ԭ��: ��
�������Ũ�������ܷ����ۻ���ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣ʵ�������������Լ���0.01 mol/L ����KMnO4��Һ��0.1 mol/L KI��Һ��3��H2O2��Һ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��.������Һ�еĽ������ӿ��ܺ���Fe2+��Fe3+�е�һ�ֻ����֣�
��.���������п��ܺ���_________�е�һ�ֻ����֡�
| | ʵ����� | Ԥ������ | ���� |
| ��֤����� | ����٣�ȡ����0.01 mol/L����KMnO4��Һ������������Һ | | |
| ����ڣ�_________ | | ����Fe3�� | |
| ��֤����� | ����������ͨ������װ�� | | ������������ |
��ʵ��̽����

���������ۡ�
��ͬѧ�����������ѡ��KSCN��Һ���������KSCN��H2O2������Һ������ɲ���������̽�����жϸ÷����Ƿ���ȷ���������ۣ�_________��
��12�֣�ij��ȤС���о�SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��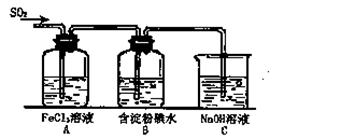
��1��SO2������Fe3+��Ӧ����Ҫ������__ __ ���������ӷ��ţ�
��2������ʵ�鷽������������ʵ������ȡ����SO2���� ��
| A��Na2SO3��Һ��HNO3 | B��Na2SO3������Ũ���� |
| C���������ڴ�����ȼ�� | D��ͭ����ŨH2SO4 |
��4�������280mL SO2���壨������Ϊ��̬������Cװ���У���C��50mL NaOH��Һ�����ʵ���Ũ������Ϊ mol/L���ܴﵽĿ�ġ�
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ��ԭ���� ��
��6���ܱ���I���Ļ�ԭ������SO2�������� ��д���й����ӷ���ʽ�� ��
