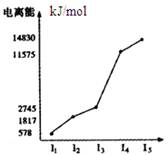
��Ŀ����
��֪A��B��C��D��E��F��G����Ԫ�����ڱ��ж���������Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�D3B�����������Ӿ�����ͬ�ĵ��Ӳ�ṹ��B��C���ɷֱ���A�γ�10�����ӷ��ӣ�B��C��ͬһ���ڣ����߿����γ������ֹ��ۻ����C��F��ͬһ���壬Bԭ��������Ӳ��p�ܼ��ϵĵ��Ӵ��ڰ���״̬��C���������������ڲ��������3����E���������������ڲ��1�����þ����Ԫ�ػش��������⣺
��1��EԪ��ԭ�ӻ�̬�����Ų�ʽ ��
��2���õ����Ų�ͼ��ʾFԪ��ԭ�ӵļ۵��ӹ��� ��
��3��F��GԪ�ض�Ӧ����ۺ����������Խ�ǿ�ķ���ʽΪ ��
��4�����Ӱ뾶D+ B3������һ������B C���縺��C F
���<������>����=������
��5��A��C�γɵ�һ����ɫ������X�й㷺Ӧ�ã�X������A��Cԭ�Ӹ�����1��1��X�ĵ���ʽΪ ����д��Cu��ϡH2SO4��X��Ӧ�Ʊ�����ͭ�����ӷ���ʽ ��
��6��д��E��D������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ ��
��1��EԪ��ԭ�ӻ�̬�����Ų�ʽ ��
��2���õ����Ų�ͼ��ʾFԪ��ԭ�ӵļ۵��ӹ��� ��
��3��F��GԪ�ض�Ӧ����ۺ����������Խ�ǿ�ķ���ʽΪ ��
��4�����Ӱ뾶D+ B3������һ������B C���縺��C F
���<������>����=������
��5��A��C�γɵ�һ����ɫ������X�й㷺Ӧ�ã�X������A��Cԭ�Ӹ�����1��1��X�ĵ���ʽΪ ����д��Cu��ϡH2SO4��X��Ӧ�Ʊ�����ͭ�����ӷ���ʽ ��
��6��д��E��D������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ ��
��1��1s22s22p63s23p1��2�֣�
��2��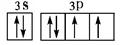 ��2�֣�
��2�֣�
��3��HClO4��2�֣�
��4������1�֣� �� ��1�֣� ����1�֣�
��5��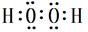 ��2�֣� Cu+2H++H2O2=Cu2++2H2O��2�֣�
��2�֣� Cu+2H++H2O2=Cu2++2H2O��2�֣�
��6��2Al+2NaOH+2H2O=2NaAlO2+3H2����2�֣�
��2��
 ��2�֣�
��2�֣���3��HClO4��2�֣�
��4������1�֣� �� ��1�֣� ����1�֣�
��5��
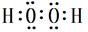 ��2�֣� Cu+2H++H2O2=Cu2++2H2O��2�֣�
��2�֣� Cu+2H++H2O2=Cu2++2H2O��2�֣���6��2Al+2NaOH+2H2O=2NaAlO2+3H2����2�֣�
���������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���H��B��N��C��O��D��Na��E��Al��F��S��G��Cl����1��EԪ��ԭ�ӻ�̬�����Ų�ʽ1s22s22p63s23p1���𰸣�1s22s22p63s23p1����2���õ����Ų�ͼ��ʾFԪ�ؼ�SԪ��ԭ�ӵļ۵��ӹ���
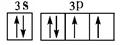 ���𰸣�
���𰸣� ����F ��GԪ�ض�Ӧ����ۺ�����H2SO4��HClO4�����Խ�ǿ�ķ���ʽΪHClO4���𰸣�HClO4���ȵ��Ӳ�ṹ��ͬ�����ӣ�ԭ������Խ��뾶ԽС��Na��<N3�D����һ������N>O��ͬ������ϵ��£��縺�Լ������縺�ԣ�O>S����;��������������H��O�γɵ�H2O2 �ĵ���ʽ��
����F ��GԪ�ض�Ӧ����ۺ�����H2SO4��HClO4�����Խ�ǿ�ķ���ʽΪHClO4���𰸣�HClO4���ȵ��Ӳ�ṹ��ͬ�����ӣ�ԭ������Խ��뾶ԽС��Na��<N3�D����һ������N>O��ͬ������ϵ��£��縺�Լ������縺�ԣ�O>S����;��������������H��O�γɵ�H2O2 �ĵ���ʽ��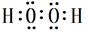 ��Cu��ϡH2SO4��H2O2��Ӧ�Ʊ�����ͭ�����ӷ���ʽ Cu+2H++H2O2=Cu2++2H2O����;
��Cu��ϡH2SO4��H2O2��Ӧ�Ʊ�����ͭ�����ӷ���ʽ Cu+2H++H2O2=Cu2++2H2O����; 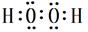 Cu+2H++H2O2=Cu2++2H2O����E��Al����NaOH��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
Cu+2H++H2O2=Cu2++2H2O����E��Al����NaOH��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 1��W����������X��Y��Z��M����Ԫ��������֮�͵�1/2 ������˵����ȷ����( )
1��W����������X��Y��Z��M����Ԫ��������֮�͵�1/2 ������˵����ȷ����( )