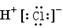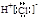��Ŀ����
��15�֣�ij����Һ������Fe2+��Fe3+��Ag+��Ba2+��Al3+��Ca2+��NH4+�����ӣ�����������ʵ�飨�����ᡢ�NH3ˮ��Br2ˮ���ǹ����ģ���

����ʵ������
��1���ж�����Һ������Ba2+��Ca2+���ӣ���д�����ɡ���_________��
��2��д����ѧʽ������A_________������D_________������E_________��
��3��д���������ӷ�Ӧ����ʽ��
��ˮ������ҺA�е�ij���ӣ�_________��
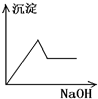
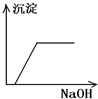
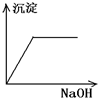
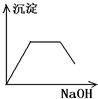
����C��������NaOH��Һ������һ�ֳ����ܽ⣺_________��

����ʵ������
��1���ж�����Һ������Ba2+��Ca2+���ӣ���д�����ɡ���_________��
��2��д����ѧʽ������A_________������D_________������E_________��
��3��д���������ӷ�Ӧ����ʽ��
��ˮ������ҺA�е�ij���ӣ�_________��
����C��������NaOH��Һ������һ�ֳ����ܽ⣺_________��
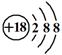
��1������Ba2+��Ca2+�����е�һ�ֻ����֣���ΪBaSO4������ˮ��CaSO4����ˮ��2�� A��AgCl�� D��Fe��OH��3�� E ��Al��OH��3
��3�� 2Fe2++Br2=2Fe3++2Br-?��Al��OH��3+OH��=AlO2��+2H2O
��3�� 2Fe2++Br2=2Fe3++2Br-?��Al��OH��3+OH��=AlO2��+2H2O
����A���ж�ΪAgCl������Ag+�Ѵ���Һ�г�������ҺA������Fe2+�ѱ���ˮ����ΪFe3+������BaSO4������ˮ��CaSO4����ˮ�����жϳ���B�����Ƕ���֮һ��Ҳ���������ǵĻ���Al��OH��3��Fe��OH��3��������ˮ������C�����Ƕ���֮һ���������ǵĻ���������C����Al��OH��3���������NaOH��Һ������NaAlO2������ҺD���ù���NaOH��Һ����C����ʣ�����D�����жϳ���D��Fe��OH��3��HAlO2�DZ�H2CO3�������ᣬCO2�������׳���֮��������HCO3����Al��OH��3���dz���E��
AlO2��+CO2+2H2O=Al��OH��3��+HCO3��
AlO2��+CO2+2H2O=Al��OH��3��+HCO3��

��ϰ��ϵ�д�
�����Ŀ