��Ŀ����
�������ֳƼ��ѣ����DME���۵�-141.5 �棬�е�-24.9 �棬��ʯ��Һ������LPG�����ƣ�����Ϊ��21���͵����ȼ�ϡ����Ʊ�ԭ�����£�
������Ȼ�����Ʊ������ѣ�
��2CH4��g��+O2��g�� CH3OCH3��g��+H2O��g�� ∆H1
CH3OCH3��g��+H2O��g�� ∆H1
���ɺϳ����Ʊ������ѣ�
��CO��g��+2H2��g�� CH3OH��g�� ∆H2=��90.7 kJ��mol-1
CH3OH��g�� ∆H2=��90.7 kJ��mol-1
��2CH3OH��g�� CH3OCH3��g��+H2O��g�� ∆H3
CH3OCH3��g��+H2O��g�� ∆H3
�ش��������⣺
��1��������Ͷ����ѵ�ȼ���ȷֱ���890.3 kJ��mol-1��1453.0 kJ��mol-1��1molҺ̬ˮ��Ϊ��̬ˮҪ����44.0 kJ����������Ӧ���е���صĻ�ѧ�������������£�
��ѧ�� | H-H | C-O | H-O��ˮ�� | H-O������ | C-H |
E/��kJ.mol-1�� | 436 | 343 | 465 | 453 | 413 |
��∆H1��__________kJ��mol-1��∆H3��__________kJ��mol-1
��2����Ӧ�ٵĻ�ѧƽ�ⳣ������ʽΪ_____________��
�Ʊ�ԭ�����У��ں��¡����ݵ��ܱ������кϳɣ������尴n��CH4��:n��O2����2��1��ϣ�����ȷ��ӳ��Ӧ����CH4������������¶ȱ仯��������_______________��

�����ܱ�����Ӧ�ٴﵽ��ѧƽ��״̬����________��
a�����������ܶȲ���
b����Ӧ�����ж����ѵİٷֺ�������
c����Ӧ��ķ�Ӧ������������ķ�Ӧ����֮�ȵ��ڻ�ѧ������֮��
d����������ѹǿ����
��3������ģ���Ʊ�ԭ������500Kʱ��2L���ܱ������г���2molCO��6molH2��8min�ﵽƽ�⣬ƽ��ʹCO��ת����Ϊ80%��c��CH3OCH3��=0.3mol��L-1����H2��ʾ��Ӧ�ڵ�������___________�����淴Ӧ�۵�ƽ�ⳣ��K3=_____________������500Kʱ�����������n��CH3OH��=n��CH3OCH3������ʱ��Ӧ��v������_________v���棩��˵��ԭ��___________��
 ������ϵ�д�
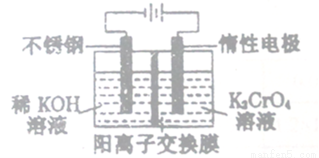
������ϵ�д����������NOx���Ǵ�����Ⱦ��֮һ����ҵ����һ���¶Ⱥʹ�����������NH3��NOx��ԭ����N2��ijͬѧ��ʵ�����ж�NH3��NOx��Ӧ������̽�����ش��������⣺
��1���������Ʊ�
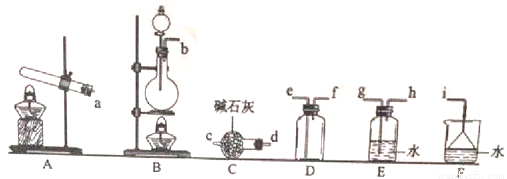
�ٰ����ķ���װ�ÿ���ѡ����ͼ�е�_________����Ӧ�Ļ�ѧ����ʽΪ_______________��
��Ԥ�ռ�һƿ����İ�����ѡ����ͼ�е�װ�ã�������˳��Ϊ������װ�á�______��������������Сд��ĸ��ʾ����
��2����������������ķ�Ӧ
�������ռ�����NH3����ע����X�У�Ӳ�ʲ�����Y�м�����������������NO2�������ü���K1��K2�кã�����һ���¶��°�ͼʾװ�ý���ʵ�顣

�������� | ʵ������ | ����ԭ�� |
��K1���ƶ�ע����������ʹX�е����建��ͨ��Y���� | ��Y����_____________ | �ڷ�Ӧ�Ļ�ѧ����ʽ ____________ |
��ע���������˻�ԭ�����̶�����װ�ûָ������� | Y����������ˮ�� | ���ɵ���̬ˮ���� |
��K2 | ��_______________ | ��______________ |



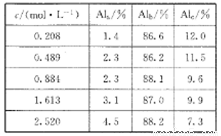
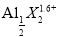 ��X��ʾ��ɫ����
��X��ʾ��ɫ����  ��ʾ��ɫ���ʣ�ͨ����ɫ�����õ�25 ��ʱAl3+Ũ����ʱ��ı仯��ϵ��ͼ��ʾ����ʼʱX��Ũ��Ϊ0.194mol��L-1����
��ʾ��ɫ���ʣ�ͨ����ɫ�����õ�25 ��ʱAl3+Ũ����ʱ��ı仯��ϵ��ͼ��ʾ����ʼʱX��Ũ��Ϊ0.194mol��L-1����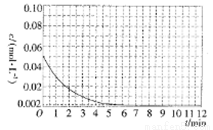
 ��Ũ��Ϊ___________��
��Ũ��Ϊ___________��