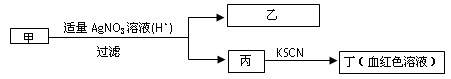
题目内容
(5分)现有一瓶浓度为0.2 mol/L的某酸溶液,可能为醋酸、盐酸、硫酸中的一种。为了确定该酸溶液的组成进行实验:取25.00 mL0.1 mol/L的氢氧化钠溶液,逐滴加入该酸溶液,恰好反应完全时所需该酸溶液体积为12.50 mL。请回答:
(1)该酸不可能是 ;
(2)用pH试纸测得反应后所得溶液呈碱性,根据此现象说明该酸溶液为 ,用离子方程式说明溶液呈碱性的原因 ;
(3)实验中滴定曲线如右图,在B点,a 12.5(填大于、小于或等于)在C点各离子浓度由大到小排序 。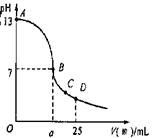
(1)该酸不可能是 ;
(2)用pH试纸测得反应后所得溶液呈碱性,根据此现象说明该酸溶液为 ,用离子方程式说明溶液呈碱性的原因 ;
(3)实验中滴定曲线如右图,在B点,a 12.5(填大于、小于或等于)在C点各离子浓度由大到小排序 。

(1)硫酸(1分)
(2)醋酸,CH3COO_ + H2O CH3COOH + OH_ (各1分)
CH3COOH + OH_ (各1分)
(3)大于 , c(CH3COO-) > c(Na +) > c(H+) > c(OH-)(2分)
(2)醋酸,CH3COO_ + H2O
 CH3COOH + OH_ (各1分)
CH3COOH + OH_ (各1分)(3)大于 , c(CH3COO-) > c(Na +) > c(H+) > c(OH-)(2分)
略

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
 稀硝酸
稀硝酸