��Ŀ����
��1��(8��) ij��ɫ����Һ���ܴ���Na����Fe3����Ba2����NO3����CO32����HCO3����SO42���еļ������ӣ��������²�����
��ȡ��������Һ����CaCl2��Һ�����������μ����������ɫ��ζ�����塣
����ȡ����Һ�μ�һ������NaOH��Һ�а�ɫ�������ɡ�
�Իش��������⣺
�� ����Һ��һ�����ڵ�������__________��һ�������ڵ�����__________��
�� ��һ�����ӷ���ʽ��ʾ�����ڵ�ʵ������
______________________________________________________��
��2����4�֣����и����е�������������Һ�еķ�Ӧ������ͬһ���ӷ���ʽ��ʾ���ǣ� ������д�������ӷ�Ӧ����ʽ��___________________________ __________________��
__________________��
| A��������ռ���Һ�����������������Һ |
B���� ����Һ�����ᡢʯ��ʯ������ ����Һ�����ᡢʯ��ʯ������ |
| C���Ȼ�����Һ����������Һ�����ᱵ��Һ������ |
| D��̼�����Һ������������Һ��̼�����Һ������������Һ |
(10��) ��2��/�գ�
(1)���� Ba2����HCO3��������Fe3����CO32����SO42��
�� Ba2��+ HCO3��+ OH��= BaCO3��+ H2O��
��Ba2��+ 2HCO3��+ 2OH��= BaCO3��+ CO32��+ 2H2O
��2����C Ba2��+ SO42��= BaSO4��
����

 ��������ܸ�ϰϵ�д�
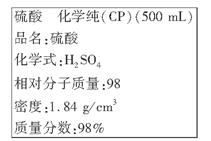
��������ܸ�ϰϵ�д�(8��)���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
(1)ȷ����8.2 g�������������������ʵ���Ʒ�����500 mL������Һ������ʱ����Ʒ�ɷ���________(������ĸ)������
A��С�ձ��С�������B���ྻֽƬ�ϡ�������C��������
(2)�ζ�ʱ����0.2000 mol��L��1���������ζ�������Һ������ѡ��________(������ĸ)��ָʾ����
A�����ȡ��� B��ʯ���
C����̪���� D������
(3)�ζ������У��۾�Ӧע��______________��������̨�ϵ�һ�Ű�ֽ����Ŀ����________________________________________________________________________
________________________________________________________________________��
(4)�����±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����________mol��L��1���ռ���Ʒ�Ĵ�����________��
|
����� |
������Һ ���(mL) |
������� |
|
|
�ζ�ǰ�Ŀ̶� (mL) |
�ζ���Ŀ̶� (mL) |
||
|
��һ�� |
10.00 |
0.40 |
20.50 |
|
�ڶ��� |
10.00 |
4.10 |
24.00 |
(5)����ʵ�������Եζ��������ʲôӰ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)?
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����________��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00 mL����Һ����ζ����________��