��Ŀ����
�������⣨H2O2����ˮ��Һ�׳�˫��ˮ���������������ǵ�����������Ϊ����ɫ������������1��д��H2O2�ĵ���ʽ ��������ڴ��� ���� ����
��2������ΪH2O2����Ϊ����ɫ������������Ҫԭ���� ��
��3����H2O2���Ӿ�����ͬ��������˫ԭ�ӵ��ʷ���Ϊ��д��ѧʽ�� ����H2O2���Ӿ�����ͬ����������ԭ�ӻ��������Ϊ��д��ѧʽ�� ��
��4��ij��ҵ��ˮ�к���һ����������Ϊ�˳�ȥ������������H2O2�����ȼ���д���÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ��H2O2�� ����
��5����˫��ˮ�����������ữ��FeCl2��Һ�У���Һ��dz��ɫ��Ϊ�ػ�ɫ��д���÷�Ӧ�����ӷ���ʽΪ ����Ӧ��H2O2�� ����
��6��˫��ˮ��ʹ���Ը��������Һ��ɫ���䷴Ӧ�����ӷ���ʽ���£��뽫������ʵĻ�ѧ����������ѧʽ��д�ڷ����
MnO4-+ H2O2+ H+�T Mn2++ H2O+ ��
��ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr��OH��3��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2��O2
��1���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2���練Ӧת����0.3mol���ӣ�������������ڱ�״�������Ϊ ��
���𰸡���������1�����ݵ���ʽ����д������д����ʽ������ԭ�����������������Ų�����ͬ�ǽ���Ԫ��֮���γɼ��Թ��ۼ���ͬ�ַǽ���Ԫ��֮���γɷǼ��Թ��ۼ���
��2��H2O2�Ļ�ԭ������ˮ��
��3�����ݵ�������ͬ���⣻
��4����������ǿ�����ԣ��ܰ�˫��ˮ��������������ˮ��
��5��˫��ˮ���������ԣ��ܰ����������������������ӣ�
��6��������ԭ��Ӧ�е�ʧ��������ȣ�
��1��˫��ˮ����ԭ�������Ը�������������
��2�����ݷ���ʽ�з�Ӧ��ת��6�����ӽ��⣮
����⣺��1��˫��ˮ��һ�ֺ����������ۼ�������ۼ��ļ��Է��ӣ�����ʽΪ��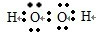 ���������γɼ��Լ����������γɷǼ��Լ����ʴ�Ϊ��
���������γɼ��Լ����������γɷǼ��Լ����ʴ�Ϊ�� �����ԣ��Ǽ��ԣ�
�����ԣ��Ǽ��ԣ�
��2��H2O2�Ļ�ԭ������ˮ������Ⱦ���ʴ�Ϊ����ԭ����Ϊˮ���Ի�������Ⱦ��
��3��˫��ˮ�к���18�����ӣ�������H2O2���Ӿ�����ͬ��������˫ԭ�ӵ��ʷ���ΪF2������H2O2���Ӿ�����ͬ����������ԭ�ӻ��������ΪH2S���ʴ�Ϊ��F2��H2S��
��4����������ǿ�����ԣ��ܰ�˫��ˮ��������������ˮ����Ӧ�ķ���ʽ��Cl2+H2O2=2HCl+O2����
�ʴ�Ϊ��Cl2+H2O2=2HCl+O2������ԭ��
��5��˫��ˮ���������ԣ��ܰ����������������������ӣ���Ӧ�ķ���ʽ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O����������
��6��˫��ˮ����Ԫ�صĻ��ϼ۴�-1�����ߵ�0�ۣ�ʧȥ1�����ӣ��������������Ԫ�صĻ��ϼ۴�+7�۽��͵�+2�ۣ��õ�2�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ�ӦΪ2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����
�ʴ�Ϊ��2��5��6��2��8��5O2����
��1��˫��ˮ���������Ĺ����DZ������Ĺ��̣���˫��ˮ����ԭ�������Ը���������������˷�Ӧ�ķ���ʽ��3H2O2+2H2CrO4=3O2��+2Cr��OH��3+2H2O���ʴ�Ϊ��3H2O2+2H2CrO4=3O2��+2Cr��OH��3+2H2O��
��2�����ݷ���ʽ��֪����Ӧ��ת��6�����ӣ������練Ӧת����0.3mol���ӣ��������������0.15mol����״���µ������0.15mol×22.4L/mol=3.36L���ʴ�Ϊ��3.36L��
���������⿼��˫��ˮ�����ʣ���Ŀ�ѶȲ���ע���õ�ʧ��������Ƚ�������ԭ��Ӧ����ʽ����ƽ��
��2��H2O2�Ļ�ԭ������ˮ��
��3�����ݵ�������ͬ���⣻
��4����������ǿ�����ԣ��ܰ�˫��ˮ��������������ˮ��
��5��˫��ˮ���������ԣ��ܰ����������������������ӣ�
��6��������ԭ��Ӧ�е�ʧ��������ȣ�
��1��˫��ˮ����ԭ�������Ը�������������
��2�����ݷ���ʽ�з�Ӧ��ת��6�����ӽ��⣮
����⣺��1��˫��ˮ��һ�ֺ����������ۼ�������ۼ��ļ��Է��ӣ�����ʽΪ��
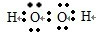 ���������γɼ��Լ����������γɷǼ��Լ����ʴ�Ϊ��
���������γɼ��Լ����������γɷǼ��Լ����ʴ�Ϊ�� �����ԣ��Ǽ��ԣ�
�����ԣ��Ǽ��ԣ���2��H2O2�Ļ�ԭ������ˮ������Ⱦ���ʴ�Ϊ����ԭ����Ϊˮ���Ի�������Ⱦ��
��3��˫��ˮ�к���18�����ӣ�������H2O2���Ӿ�����ͬ��������˫ԭ�ӵ��ʷ���ΪF2������H2O2���Ӿ�����ͬ����������ԭ�ӻ��������ΪH2S���ʴ�Ϊ��F2��H2S��
��4����������ǿ�����ԣ��ܰ�˫��ˮ��������������ˮ����Ӧ�ķ���ʽ��Cl2+H2O2=2HCl+O2����
�ʴ�Ϊ��Cl2+H2O2=2HCl+O2������ԭ��
��5��˫��ˮ���������ԣ��ܰ����������������������ӣ���Ӧ�ķ���ʽ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O����������
��6��˫��ˮ����Ԫ�صĻ��ϼ۴�-1�����ߵ�0�ۣ�ʧȥ1�����ӣ��������������Ԫ�صĻ��ϼ۴�+7�۽��͵�+2�ۣ��õ�2�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ�ӦΪ2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����
�ʴ�Ϊ��2��5��6��2��8��5O2����
��1��˫��ˮ���������Ĺ����DZ������Ĺ��̣���˫��ˮ����ԭ�������Ը���������������˷�Ӧ�ķ���ʽ��3H2O2+2H2CrO4=3O2��+2Cr��OH��3+2H2O���ʴ�Ϊ��3H2O2+2H2CrO4=3O2��+2Cr��OH��3+2H2O��
��2�����ݷ���ʽ��֪����Ӧ��ת��6�����ӣ������練Ӧת����0.3mol���ӣ��������������0.15mol����״���µ������0.15mol×22.4L/mol=3.36L���ʴ�Ϊ��3.36L��
���������⿼��˫��ˮ�����ʣ���Ŀ�ѶȲ���ע���õ�ʧ��������Ƚ�������ԭ��Ӧ����ʽ����ƽ��

��ϰ��ϵ�д�
�����Ŀ
�ش������йع�������øʵ������⣺
��1��Ϊ��֤������ø���и�Ч�ԣ�ͬѧ�����������ʵ�飺
��ָ��������֮�������и����� ��
��B���Թ���û�����ݲ�����ԭ���ǣ� ��
��2���¶ȶ�ø������Ӱ�죮��һ������£������ù�������øΪ����̽���¶ȶ�ø���Ե�Ӱ�죬ԭ���� ����̽���¶ȶ�ø���Ե�Ӱ��ʱ��һ��ѡ�æ�-����ø��ʵ����ϣ�д��̽����-����ø�����¶ȵ�ʵ��˼· ʵ�������һ���õ�Һ���������Ƿֽ⣬����������Լ���ⷴӦ���ԭ���� ��
��1��Ϊ��֤������ø���и�Ч�ԣ�ͬѧ�����������ʵ�飺
| �Թܱ�� | A | B | |
| ���� | 1 | 5mL����������Һ | 5mL����������Һ |
| 2 | 5�ι�������ø��Һ | 5�� H2O | |
| 3 | �����ݲ��� | �����ݲ��� | |
��B���Թ���û�����ݲ�����ԭ���ǣ�
��2���¶ȶ�ø������Ӱ�죮��һ������£������ù�������øΪ����̽���¶ȶ�ø���Ե�Ӱ�죬ԭ����
