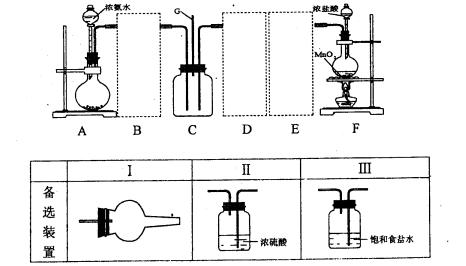
��Ŀ����
��11�֣�������������װ�ü��Լ���װһ��װ�á��������ǣ�����ȡ���������Cl2�����ռ���������������Cl2�ͳ�ʪ��Cl2����Ư���ԡ��Իش�

��1�������������������װ������������������ӿڱ�ţ��� a ��_______��______
��______ ��______ ��______ ��______ ��______ ��______ ��h��
��2����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��________________________________________ ��
��3��Dװ�õ�������____________________ ��Eװ�õ�������____________________ ��
Fװ�õ����� �ǡ�________________________��
�ǡ�________________________��
��4��Cƿ�е�������______________________ ��Bƿ�е�������___________________ ��������ʵ˵����Ư�����õ�������___________________ ��


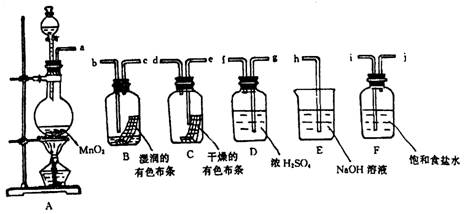
��1�������������������װ������������������ӿڱ�ţ��� a ��_______��______
��______ ��______ ��______ ��______ ��______ ��______ ��h��
��2����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��________________________________________ ��
��3��Dװ�õ�������____________________ ��Eװ�õ�������____________________ ��
Fװ�õ�����
 �ǡ�________________________��
�ǡ�________________________����4��Cƿ�е�������______________________ ��Bƿ�е�������___________________ ��������ʵ˵����Ư�����õ�������___________________ ��
��1��i��j��g��f��e��d��b��c��
��2��MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��3���������� ���ն�������� ��ȥHCl����
(4)��ɫ��������ɫ ��ɫ������ɫ ������(HClO)
��2��MnO2+4HCl(Ũ)
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O��3���������� ���ն�������� ��ȥHCl����
(4)��ɫ��������ɫ ��ɫ������ɫ ������(HClO)
��

��ϰ��ϵ�д�
�����Ŀ