��Ŀ����
�����廯����A��B��Ϊͬ���칹�壬B�Ľṹ��ʽ��CH3COO- -COOCH2CH3��A���٢ڷ�Ӧ��C��D��E��B���٢ڲ���Ӧ��E��F��H������Ӧ���̣��������ʼ����ϵ��ͼ��
-COOCH2CH3��A���٢ڷ�Ӧ��C��D��E��B���٢ڲ���Ӧ��E��F��H������Ӧ���̣��������ʼ����ϵ��ͼ��
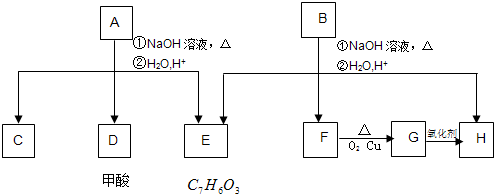
��1��E�к��й����ŵ�������
��2��A�����ֿ��ܵĽṹ����ṹ��ʽ�ֱ�Ϊ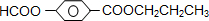
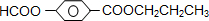 ��
��
 ��
��
��3��B��C��D��F��G�������л�Ϊͬϵ�����
��4��F��H��ŨH2SO4�����¼���ʱ������Ӧ�ķ���ʽΪ
 -COOCH2CH3��A���٢ڷ�Ӧ��C��D��E��B���٢ڲ���Ӧ��E��F��H������Ӧ���̣��������ʼ����ϵ��ͼ��
-COOCH2CH3��A���٢ڷ�Ӧ��C��D��E��B���٢ڲ���Ӧ��E��F��H������Ӧ���̣��������ʼ����ϵ��ͼ��
��1��E�к��й����ŵ�������
�ǻ����Ȼ�
�ǻ����Ȼ�
����2��A�����ֿ��ܵĽṹ����ṹ��ʽ�ֱ�Ϊ
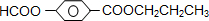
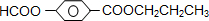


��3��B��C��D��F��G�������л�Ϊͬϵ�����
C��F
C��F
����4��F��H��ŨH2SO4�����¼���ʱ������Ӧ�ķ���ʽΪ
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O
| Ũ���� |
| �� |
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O
��| Ũ���� |
| �� |
������Bˮ�������E��F��H������B�Ľṹ��ʽ��֪EΪHO- -COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO-
-COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO- -COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ
-COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ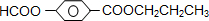 ��
�� ������л���Ľṹ�������Լ���Ŀ��Ҫ��ɽ����⣮
������л���Ľṹ�������Լ���Ŀ��Ҫ��ɽ����⣮
 -COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO-
-COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO- -COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ
-COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ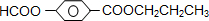 ��
�� ������л���Ľṹ�������Լ���Ŀ��Ҫ��ɽ����⣮
������л���Ľṹ�������Լ���Ŀ��Ҫ��ɽ����⣮����⣺Bˮ�������E��F��H������B�Ľṹ��ʽ��֪EΪHO- -COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO-
-COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO- -COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ
-COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ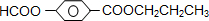 ��
�� ��
��
��1��EΪHO- -COOH�����еĹ�����Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
-COOH�����еĹ�����Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
��2�������Ϸ�����֪A�����ֿ��ܵĽṹ����ṹ��ʽ�ֱ�Ϊ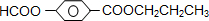 ��
�� ��
��
�ʴ�Ϊ��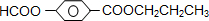 ��
�� ��
��
��3���������Ϸ�����֪��Ϊͬϵ�����C��F�����߶�Ϊ����һԪ�����ʴ�Ϊ��C��F��
��4��FΪCH3CH2OH��HΪCH3COOH��������Ũ���������·���������Ӧ��������������
��Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
 -COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO-
-COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO- -COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ
-COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ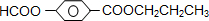 ��
�� ��
����1��EΪHO-
 -COOH�����еĹ�����Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
-COOH�����еĹ�����Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ�����2�������Ϸ�����֪A�����ֿ��ܵĽṹ����ṹ��ʽ�ֱ�Ϊ
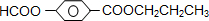 ��
�� ��
���ʴ�Ϊ��
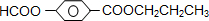 ��
�� ��
����3���������Ϸ�����֪��Ϊͬϵ�����C��F�����߶�Ϊ����һԪ�����ʴ�Ϊ��C��F��
��4��FΪCH3CH2OH��HΪCH3COOH��������Ũ���������·���������Ӧ��������������
��Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
���������⿼���л�����ƶϣ���Ŀ�ѶȲ�����ע�����Bˮ��IJ���������֮��ת���Ĺ�ϵΪͻ�ƿڽ��н��

��ϰ��ϵ�д�
�����Ŀ
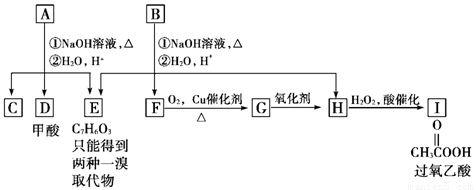
 ��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ�
��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ�
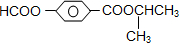
 �����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��
�����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��