��Ŀ����
(12��)50 mL 1.0 mol��L��1�����50 mL 1.1 mol��L��1����������Һ����ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȡ��Իش��������⣺
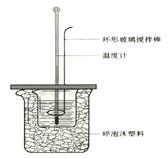
�Ŵ�С�ձ�������ܡ��������β����������Ϊ���ν�������ͭ������ԭ���� ��
�Ǵ��ձ����粻������ĭ���ϵ����� ��
�� ����ܡ���Ӳֽ�壬������к�����ֵ��Ӱ���� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
���������60 mL 1.0 mol��L��1�����50 mL 1.1 mol��L��1����������Һ���з�Ӧ����������ʵ����ȣ��������� �������ӡ��������١����䡱���������к�����ֵ �������ӡ��� �����١��� �����䡱����

�Ŵ�С�ձ�������ܡ��������β����������Ϊ���ν�������ͭ������ԭ���� ��
�Ǵ��ձ����粻������ĭ���ϵ����� ��
�� ����ܡ���Ӳֽ�壬������к�����ֵ��Ӱ���� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
���������60 mL 1.0 mol��L��1�����50 mL 1.1 mol��L��1����������Һ���з�Ӧ����������ʵ����ȣ��������� �������ӡ��������١����䡱���������к�����ֵ �������ӡ��� �����١��� �����䡱����
��1������ʵ������е�������ʧ
��2�� ���� ��������� ��3��ƫ�� ��4������ ����
��2�� ���� ��������� ��3��ƫ�� ��4������ ����
�ڷ�Ӧ�б��뾡���ܵļ�����������ʧ�������������ȵ������塣���Բ��ܽ����β����������Ϊ���ν�����������Ӳֽ�壬�������������ʧ��ʹ���ƫ�͡���Ϊ��ͼ�����ʵ��������ˣ����Էų�������Ҫ���ӣ����к��Ȳ��䡣

��ϰ��ϵ�д�
�����Ŀ
 2NH3��g����H����92.0 kJ/mol������ͬ�¶��£����ܱ�������ͨ��1/2 mol N2��3/2 mol H2���ﵽƽ��ʱ�ų�46.0 kJ������
2NH3��g����H����92.0 kJ/mol������ͬ�¶��£����ܱ�������ͨ��1/2 mol N2��3/2 mol H2���ﵽƽ��ʱ�ų�46.0 kJ������
 2NH3(g)+CO2(g) �ڲ�ͬ�¶��£��÷�Ӧƽ��״̬�������ݼ��ұ�������˵����ȷ����
2NH3(g)+CO2(g) �ڲ�ͬ�¶��£��÷�Ӧƽ��״̬�������ݼ��ұ�������˵����ȷ����  ��������������ˮʾ��ͼ����ͼ��ʾ�����������������ӽ���Ĥ������������������ͨ����
��������������ˮʾ��ͼ����ͼ��ʾ�����������������ӽ���Ĥ������������������ͨ����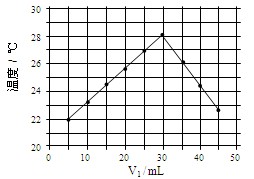
 2H2(g)+O2(g)�����Զ����������Ǹ����о�����Ҫ�ȵ㡣����Ϊ�����о�����������
2H2(g)+O2(g)�����Զ����������Ǹ����о�����Ҫ�ȵ㡣����Ϊ�����о�����������