��Ŀ����
10������˵����ȷ���ǣ�������| A�� | ��${\;}_{10}^{20}$Ne��${\;}_{10}^{22}$Ne����������ͬ������${\;}_{10}^{20}$Ne��${\;}_{10}^{22}$Ne��Ϊͬλ�� | |
| B�� | N2��g��+3H2��g��?2NH3��g����H��0��������������ʱ�����¶ȣ���Ӧ����v��H2����������ƽ��ת���ʾ����� | |
| C�� | ������������ˮ���õ���Һ�ɵ��磬˵�����������ǵ���� | |
| D�� | C2H6�����мȺ����Լ��ֺ��Ǽ��Լ� |
���� A��������=������-��������
B�������¶ȣ�ƽ�������ƶ���
C����ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ�
D�������к�C-C��C-H����
��� �⣺A��������=������-��������${\;}_{10}^{20}$Ne��${\;}_{10}^{22}$Ne���������ֱ�Ϊ��10��12����A����
B�������¶ȣ�ƽ�������ƶ�������ת���ʼ�С����B����
C�����������ˮ��Ӧ���������ᣬ�������ܵ���������ƶ����������ӣ����Զ��������ˮ��Һ���磬����������ӵ�������������Ƕ����������Զ��������Ƿǵ���ʣ���C����
D�������к�C-C��C-H������C2H6�����мȺ����Լ��ֺ��Ǽ��Լ�����D��ȷ��
��ѡD��
���� ���⿼������������������������֮������ϵ����ѧƽ�⡢�ǵ�����Լ���ѧ���ȣ��ѶȲ���ע����ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ�������������������У�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
18��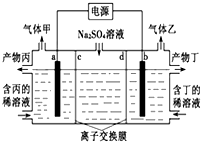 �����������Һ����������ռ���Һ��װ������ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ1��2������˵����ȷ���ǣ�������
�����������Һ����������ռ���Һ��װ������ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ1��2������˵����ȷ���ǣ�������
 �����������Һ����������ռ���Һ��װ������ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ1��2������˵����ȷ���ǣ�������
�����������Һ����������ռ���Һ��װ������ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ1��2������˵����ȷ���ǣ�������| A�� | a�����Դ�ĸ������� | B�� | a�缫��Ӧʽ��2H2O+2e-=H2��+2OH- | ||
| C�� | ���ӽ���ĤdΪ�����ӽ���Ĥ | D�� | �����Ϊ������Һ |
19�����и�����������ͬ���칹����ǣ�������
| A�� | ���ʯ��ʯī | B�� | ����������������� | ||
| C�� |  �� �� | D�� | ����ϩ��ͼ������ |
20���ڼ�����Һ���ܴ�����������ҺΪ��ɫ�����������ǣ�������
| A�� | K+��MnO4-��Na+��Cl- | B�� | Fe3+��Na+��Cl-��SO42- | ||
| C�� | NH4+��Na+��NO3-��CO32- | D�� | Na+��K+��SiO32-��NO3- |
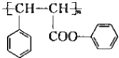 ����һ�ֻ����͵���Ϳ�ϣ���ϳ�·������ͼ��ʾ��
����һ�ֻ����͵���Ϳ�ϣ���ϳ�·������ͼ��ʾ��
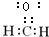 ��E�Ǹ߷��ӻ�̨���ṹ��ʽΪ
��E�Ǹ߷��ӻ�̨���ṹ��ʽΪ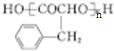 ��
��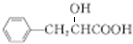 $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$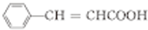 +H2O��
+H2O��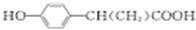 ��д������һ�ֵĽṹ��ʽ����
��д������һ�ֵĽṹ��ʽ���� Cl02������һ�ֳ��õ����������ҹ���2000��������Cl02��������������ˮ����������������������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ���������ϡ�ͣ�ͬʱ��Ҫ������ա�����ȣ�
Cl02������һ�ֳ��õ����������ҹ���2000��������Cl02��������������ˮ����������������������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ���������ϡ�ͣ�ͬʱ��Ҫ������ա�����ȣ�