��Ŀ����
����ѡһ������ѧ�뼼����
�ۺ��Ȼ�������Ļ�ѧʽΪ[Al2(OH)nCl6��n��XH2O]m������һ�ָ�Ч��ˮ�������������Ʊ�ԭ���ǵ�������AlCl3��Һ��pH��ͨ���ٽ���ˮ����ᾧ���������Ʊ�ԭ����Ҫ�����ӹ���ҵ�ķ���--���ң�����Ҫ��Al2O3��Al������SiO2�����ʡ��ۺ��Ȼ��������Ĺ����������£�
�ۺ��Ȼ�������Ļ�ѧʽΪ[Al2(OH)nCl6��n��XH2O]m������һ�ָ�Ч��ˮ�������������Ʊ�ԭ���ǵ�������AlCl3��Һ��pH��ͨ���ٽ���ˮ����ᾧ���������Ʊ�ԭ����Ҫ�����ӹ���ҵ�ķ���--���ң�����Ҫ��Al2O3��Al������SiO2�����ʡ��ۺ��Ȼ��������Ĺ����������£�
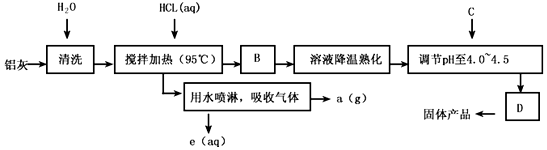
��1��������Ȳ��������з�����Ӧ�����ӷ���ʽΪ��__________��_____________��
��2������������B��D�IJ������Ʒֱ���_________��_________��B��D��Ϊ��������
��3����Ӧ�и���Ʒa��_________________��
��4�����������п�ѭ��ʹ�õ�������_____________���û�ѧʽ��ʾ����
��5������pH��4��0��4��5��Ŀ����_____________��
��6��ʵ����Ҫ�ⶨˮ��������Ʒ��n��x��ֵ��Ϊʹ�ⶨ�����ȷ����õ��ľ���ϴ�������������C���ʿ�ѡ��__________��
A��NaOH B��Al C����ˮ D��Al2O3
��2������������B��D�IJ������Ʒֱ���_________��_________��B��D��Ϊ��������
��3����Ӧ�и���Ʒa��_________________��
��4�����������п�ѭ��ʹ�õ�������_____________���û�ѧʽ��ʾ����
��5������pH��4��0��4��5��Ŀ����_____________��
��6��ʵ����Ҫ�ⶨˮ��������Ʒ��n��x��ֵ��Ϊʹ�ⶨ�����ȷ����õ��ľ���ϴ�������������C���ʿ�ѡ��__________��
A��NaOH B��Al C����ˮ D��Al2O3
��1��Al2O3+6H+=2Al3++3H2O��2Al+6H+=2Al3++3H2��
��2�����ˣ�����
��3��H2
��4��HCl
��5���ٽ�AlCl3ˮ�⣬ʹ��������
��6��BD
��2�����ˣ�����
��3��H2
��4��HCl
��5���ٽ�AlCl3ˮ�⣬ʹ��������
��6��BD

��ϰ��ϵ�д�
�����Ŀ
 CH3CH2Cl��HCl
CH3CH2Cl��HCl  CH3CH2Cl
CH3CH2Cl 



