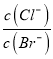��Ŀ����
����Ŀ��������(��Ҫ�ɷ���Al2O3��������SiO2��Fe2O3����)�ǹ�ҵ����ȡ����ԭ�ϡ�ʵ����ģ�ҵ����������Ϊԭ����ȡ��ȡAl2(SO4)3�����������[NH4Al(SO4)2`12H2O]�Ĺ���������ͼ��ʾ��

��ش��������⣺
(1)����a�Ļ�ѧʽΪ_________�����з�Ӧ�����ӷ���ʽΪ___________________________��
(2)���з�Ӧ�Ļ�ѧ����ʽΪ__________________________�����������Һ�л������������ʵ���������Ϊ(���������)___________����ȴ�ᾧ������ϴ�Ӹ���ȡ�
(3)��1000 kg��������36%��������Ϊԭ����ȡAl2(SO4)3����������������98%������(�ܶ�1.84 g��cm-1)___________L(����һλС��)��
(4)��ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1��1����Ͷ��ʱ�������е�Al2O3��H2SO4�����ʵ���֮��Ϊ_____________��
���𰸡�SiO2 [A1(OH)4]-+CO2=Al(OH)3��+HCO3-��A1O2-+CO2+2H2O=HCO3-+ Al(OH)3�� A12O3+4H2SO4+2NH3=2NH4Al(SO4)2+3H2O ����Ũ�� 575.4 3��10
��������
��1��SiO2���������ᡣ����NaAlO2��Һͨ��CO2����Al��OH��3������
��2��Al2O3��ϡ���ᡢ������Ӧ�����������Һ����ô��ᾧˮ�ľ���ķ���������Ũ������ȴ�ᾧ������ϴ�ӣ�
��3����V��H2SO4��=![]() �Լ� c��H2SO4��=
�Լ� c��H2SO4��=![]() �ɽ⣻
�ɽ⣻
��4������Al3+��SO42-�غ�ԭ���ɵá�
��1����������Al2O3��Fe2O3���������ᣬSiO2���������ᣬ���Թ���a�Ļ�ѧʽΪSiO2��Al2O3�����ռ�����NaAlO2��Һ��������ͨ��CO2����Al��OH��3��������Ӧ�����ӷ���ʽΪA1O2-+CO2+2H2O=HCO3-+ Al(OH)3����
��2��Al��OH��3�ֽ�����Al2O3��Al2O3��ϡ���ᡢ������Ӧ�����������Һ����Ӧ�Ļ�ѧ����ʽΪA12O3+4H2SO4+2NH3=2NH4Al(SO4)2+3H2O�����������Һ�л������������ʵ���������Ϊ����Ũ������ȴ�ᾧ������ϴ�ӣ�
��3��m��Al2O3��=1 000 kg��36%=360 kg��n��Al2O3��=![]() �����ݣ�Al2O3+3H2SO4=Al2��SO4��3+3H2O��n��H2SO4��=
�����ݣ�Al2O3+3H2SO4=Al2��SO4��3+3H2O��n��H2SO4��=![]() ��c��H2SO4��=
��c��H2SO4��=![]() =
=![]() =18.4mol/L����������������98%������V��H2SO4��=
=18.4mol/L����������������98%������V��H2SO4��=![]() 575.4L��
575.4L��
��4�����Ƶõ�Al2��SO4��3��NH4Al(SO4)2`12H2O�����ʵ�������1 mol����Al3+��3 mol��SO42-��5 mol������Al3+��SO42-�غ�ԭ���ɵã�����Al2O3��H2SO4�����ʵ���֮��Ϊ��![]() =3��10��
=3��10��