��Ŀ����
���״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϣ����㷺�����л��ϳɡ�ҽҩ��ũҩ��Ϳ�ϡ�Ⱦ�ϡ����������ȹ�ҵ�С���ҵ��һ���ں����ܱ������в������з�Ӧ�ϳɼ״���
CO(g) 2H2(g)
2H2(g)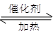 CH3OH(g)
CH3OH(g) Q
Q
�±����������Ƿ�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
|
�¶� |
250�� |
300�� |
350�� |
|
K |
2.041 |
0.270 |
0.012 |
��1���жϷ�Ӧ�ﵽƽ��״̬�������ǣ� ��
A�����������ܶȲ���
B����������ƽ����Է�����������
C������CH3OH������������CO���������
D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯
��2���ɱ��������ж�Q
0 ����������� ����������Ҫ���COת���ʣ��ɲ�ȡ�Ĵ�ʩ
����������Ҫ���COת���ʣ��ɲ�ȡ�Ĵ�ʩ
�ǣ� ��
A��������� B������CO C������H2 D������
��3��ij�¶��£���2 mol CO��6 mol H2����2 L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���c mol��L������CH3OH��ʾ�ķ�Ӧ����v
mol��L������CH3OH��ʾ�ķ�Ӧ����v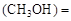 mol /(L��min)��
mol /(L��min)��
CO��ת����Ϊ ��
��4��ԭ��CO��H2������C��H2O��Ӧ��ȡ���漰�ķ�Ӧ����ʽ���£�
C(s) CO2(g)
CO2(g)
 2CO(g)
ƽ�ⳣ��K1
2CO(g)
ƽ�ⳣ��K1
C(s) H2O(g)
H2O(g)
 CO(g)
CO(g) H2(g)
ƽ�ⳣ��K2
H2(g)
ƽ�ⳣ��K2
CO(g) H2O(g)
H2O(g)
 H2(g)
H2(g) CO2(g)
ƽ�ⳣ��K3
CO2(g)
ƽ�ⳣ��K3
��K1��K2 ��K3֮��Ĺ�ϵ�ǣ� ��
��1�� BD (2��)
��2��>(1��)��C (1��)
��3��0.08 (1��) 80% (1��)
��4��K2=K1��K3 (2��)
��������
���������
��1��A�����ܱ�������������䣬�������壬�����غ㣬���������ܶ�ʼ�ղ��䣬������ƽ���־������B��ȷ��C����CH3OH������CO��������ͬ�����ʣ�����D��ȷ��
��2���ɱ���֪��T���ߣ�K��С������Ӧ�Ƿ��ȷ�Ӧ��Q>0�����COת���ʣ���Ӧ������У�ѡC��
��3�� CO(g)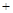 2H2(g)
2H2(g) 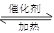 CH3OH(g)
CH3OH(g)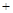 Q
Q
��Ũ�ȣ� 1 3 0
��Ũ�� 0.8 1.6 0.8
ƽ�� 0.2
v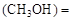 0.8/10=0.08(mol��L��min)
0.8/10=0.08(mol��L��min)
CO��ת����= 0.8 /1*100%= 80%
��4����C(s)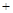 CO2(g)
CO2(g) 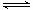 2CO(g) ƽ�ⳣ��K1
2CO(g) ƽ�ⳣ��K1
��C(s)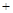 H2O(g)
H2O(g) 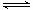 CO(g)
CO(g)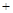 H2(g)
ƽ�ⳣ��K2
H2(g)
ƽ�ⳣ��K2
��CO(g)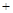 H2O(g)
H2O(g) 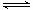 H2(g)
H2(g)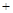 CO2(g) ƽ�ⳣ��K3
CO2(g) ƽ�ⳣ��K3
��=��+�� ����K2=K1��K3
���㣺�����Ի�������Ϊ����������ƽ�ⳣ����ƽ������֪ʶ��

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д� CH3OH(g) ��H1
CH3OH(g) ��H1  CH3OH(g)+H2O(g) ��H2
CH3OH(g)+H2O(g) ��H2 
