��Ŀ����
����Ŀ�������裨Si3N4�������������մɸ��ϲ��ϣ��ں��պ��졢��������������е���������Ź㷺��Ӧ�á���ʯӢɰ�ϳɵ������ĩ��·����ͼ��ʾ��

������NH2�и�Ԫ�صĻ��ϼ���NH3��ͬ��
��ش��������⣺
��1��ʯӢɰ������������ʹ�ͬ��ţ���NaOHΪ�����û�ѧ����ʽ��ʾ��ԭ��_________________��
��2��ͼʾ�������ı仯�У�����������ԭ��Ӧ����________________��
��3��SiCl4�ڳ�ʪ�Ŀ����о��ҷ�Ӧ���������������¹�ҵ������������������SiCl4ˮ��Ļ�ѧ����ʽΪ_______________________________________��
��4��SiCl4�Ͱ�����1400���¿�����ȡ�����裬��Ӧ��ѧ����ʽΪ___________________________��
��5����Ӧ���Ļ�ѧ����ʽΪ2C+ SiO2![]() Si+2CO�����˷�Ӧ����̼���㣬��������SiC��CO��д���ø���Ӧ�Ļ�ѧ����ʽ_____________________________________�������������ͻ�ԭ�������ʵ���֮��Ϊ__________��
Si+2CO�����˷�Ӧ����̼���㣬��������SiC��CO��д���ø���Ӧ�Ļ�ѧ����ʽ_____________________________________�������������ͻ�ԭ�������ʵ���֮��Ϊ__________��
���𰸡� SiO2��2NaOH===Na2SiO3��H2O �٢� SiCl4��3H2O===4HCl����H2SiO3����SiCl4��4H2O===4HCl����H4SiO4�� 3SiCl4+4NH3![]() Si3N4+12HCl 3C+SiO2
Si3N4+12HCl 3C+SiO2![]() SiC+2CO�� 1:2
SiC+2CO�� 1:2
�����������������������ʯӢɰ�ϳɵ������·��Ϊ���壬����SiO2�����ʡ���ѧ����ʽ����д��������ԭ��Ӧ�ķ�����
��1��ʯӢɰ����Ҫ�ɷ�ΪSiO2��SiO2�������������SiO2��������ʷ�Ӧ��SiO2��NaOH��Ӧ����Na2SiO3��H2O����Ӧ�Ļ�ѧ����ʽΪSiO2+2NaOH=Na2SiO3+H2O��
��2�����Ļ�ѧ����ʽΪSiO2+2C![]() Si+2CO�����÷�Ӧ��SiԪ�صĻ��ϼ���+4�۽���0����CԪ�صĻ��ϼ���0������+2�ۣ�Ϊ������ԭ��Ӧ�����Ļ�ѧ����ʽΪSi+2Cl2
Si+2CO�����÷�Ӧ��SiԪ�صĻ��ϼ���+4�۽���0����CԪ�صĻ��ϼ���0������+2�ۣ�Ϊ������ԭ��Ӧ�����Ļ�ѧ����ʽΪSi+2Cl2![]() SiCl4��SiԪ�صĻ��ϼ���0������+4����ClԪ�صĻ��ϼ���0�۽���-1�ۣ�Ϊ������ԭ��Ӧ����Ϊ�����仯�����Ļ�ѧ����ʽΪSiCl4+4NH3=Si��NH2��4+4HCl��-NH2�и�Ԫ�صĻ��ϼ���NH3��ͬ����Ӧǰ��Ԫ�صĻ��ϼ۲��䣬�÷�Ӧ����������ԭ��Ӧ�����Ļ�ѧ����ʽΪ3Si��NH2��4
SiCl4��SiԪ�صĻ��ϼ���0������+4����ClԪ�صĻ��ϼ���0�۽���-1�ۣ�Ϊ������ԭ��Ӧ����Ϊ�����仯�����Ļ�ѧ����ʽΪSiCl4+4NH3=Si��NH2��4+4HCl��-NH2�и�Ԫ�صĻ��ϼ���NH3��ͬ����Ӧǰ��Ԫ�صĻ��ϼ۲��䣬�÷�Ӧ����������ԭ��Ӧ�����Ļ�ѧ����ʽΪ3Si��NH2��4![]() Si3N4+8NH3����Ӧǰ��Ԫ�صĻ��ϼ۲��䣬�÷�Ӧ����������ԭ��Ӧ����~���ı仯�У�����������ԭ��Ӧ�����٢���
Si3N4+8NH3����Ӧǰ��Ԫ�صĻ��ϼ۲��䣬�÷�Ӧ����������ԭ��Ӧ����~���ı仯�У�����������ԭ��Ӧ�����٢���
��3��SiCl4�ڳ�ʪ�Ŀ����о��ҷ�Ӧ�������������¹�ҵ������������������˵��SiCl4ˮ������HCl���������SiCl4ˮ��Ļ�ѧ����ʽΪSiCl4+3H2O=H2SiO3��+4HCl����SiCl4+4H2O=H4SiO4��+4HCl����
��4��SiCl4�Ͱ�����1400���·�Ӧ����Si3N4������ԭ���غ㣬�÷�Ӧ������HCl����Ӧ�Ļ�ѧ����ʽΪ3SiCl4+4NH3![]() Si3N4+12HCl��
Si3N4+12HCl��
��5�������̼��SiO2�����·�Ӧ����SiC��CO�Ļ�ѧ����ʽΪSiO2+3C![]() SiC+2CO�����ڸ÷�Ӧ�У�CԪ�صĻ��ϼ۲�����0������CO�е�+2�ۣ�������0�۽���SiC�е�-4����C�������������ǻ�ԭ����3molC���뷴Ӧ��2mol����������ԭ����1mol����ԭ�����������������ͻ�ԭ�����ʵ���֮��Ϊ1:2��
SiC+2CO�����ڸ÷�Ӧ�У�CԪ�صĻ��ϼ۲�����0������CO�е�+2�ۣ�������0�۽���SiC�е�-4����C�������������ǻ�ԭ����3molC���뷴Ӧ��2mol����������ԭ����1mol����ԭ�����������������ͻ�ԭ�����ʵ���֮��Ϊ1:2��

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�����Ŀ��ijУ����С��Ϊ��̽��ͭ������ķ�Ӧ���������ʵ�顣
��1����ͬѧ��̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����װ����ͼ��ʾ������װ�ú̶�װ�þ�����ȥ����ͼ��KΪֹˮ�У����ڹر�״̬����F�Ǻ���һ�������ע������
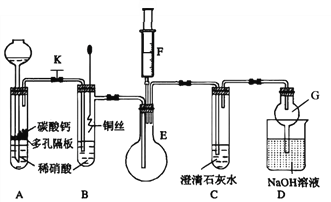
��ش��й�������
��װ��A�н��з�Ӧʱ��ֹˮ��K����װ��C��_________ʱ���ر�ֹˮ��K���Է�������������Ŀ����________��A�з�Ӧ�����ӷ���ʽΪ______________��
����������е�����������װ��B��ͭ˿����ϡ���ᣬ����֮���۲쵽װ��B�е�������__________________��B�з�Ӧ�����ӷ���ʽΪ____________________��
��Ϊ��һ��֤��������NO����ע����F�еĿ�������E�У�������������_________________��
��װ��G��������_____________________��
��2��ͬѧ�Ƿ���ͭ��ϡ��Ũ���ᷴӦ������Һ����ɫ��ͬ������¼���£�
��1 gϸͭ˿����ʢ��10 mL��1 mol��L-1 HNO3��Һ���Թ��м��� | ͭ˿��������ɫ�����ݳ�����Һ��Ϊ����ɫ |
��1 gϸͭ˿����ʢ��10 mL��14 mol��L-1HNO3��Һ���Թ��� | ������������ɫ���壬��Һ��Ϊ��ɫ����ɫ���dz��δ������ɫ |
��ͬѧ��Ϊ��ͭ��Ũ���ᷴӦ����Һ���ܽ������ɵ����壬Ҳ��ͬѧ��Ϊ����Һ��ʣ������Ũ�Ƚϴ����£�ͬѧ�Ƿֱ����������4��ʵ�����жϸÿ����Ƿ���ȷ�����·����п��е��ǣ�ѡ�������ĸ��____________��
a. ��������ɫ��Һ��ͨ�뵪�����۲���ɫ�仯
b. ��ˮϡ��������ɫ��Һ���۲���ɫ�仯
c. �͵�����ͭ��Һ�в��ϵμ�14 mol��L-1HNO3��Һ
d. ������ͭ��Һ��ͨ��Ũ������ͭ��Ӧ���������壬�۲���ɫ�仯
