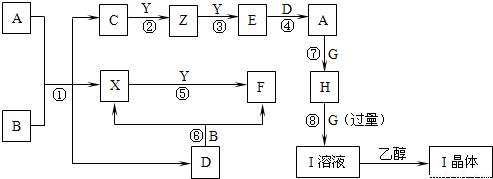
��Ŀ����

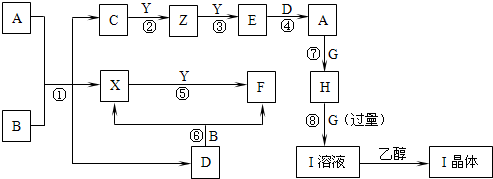
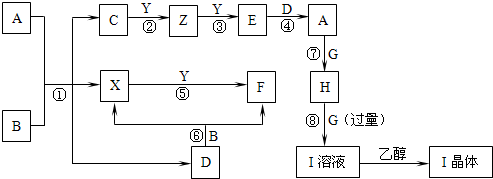
����ת����ϵ�г��ڢۢ��⣬���������Һ�н��У��Ҳ��ַ�Ӧ������������ȥ����֪X��YΪ�ǽ������ʣ�XΪ��ɫ��ĩ��ZΪ����������F��G��YΪ���壬D��һ���ᣬHΪ��ɫ��״������IΪ����ɫ���壮��ҵ�ϳ����÷�Ӧ������Z�����÷�Ӧ�ۢ��Ʊ�A��һ����C����Ӧ�ڢ���ȫ�õ�Eʱ���������䣮A��D��I��Һ�μ�BaCl2��Һ��������ɫ������B����ɫ��Ӧ�ʻ�ɫ������գ�
��1��д��C��I�Ļ�ѧʽ
Cu2S
Cu2S
����[Cu��NH3��4]SO4?H2O
��[Cu��NH3��4]SO4?H2O
����2���õ���ʽ��ʾG���γɹ���
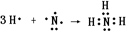
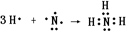
��3���ö��Ե缫���A��Һ�����ӷ���ʽΪ
2Cu2++2H2O
2 Cu+O2��+4H+
| ||
2Cu2++2H2O
2 Cu+O2��+4H+
����Ӧ�����ӷ���ʽΪ
| ||
S2O32-+2H+�TS��+SO2��+H2O
S2O32-+2H+�TS��+SO2��+H2O
����4��F�������Ư���ԣ���Ư��ԭ��Ϊ
SO2�����л�ɫ�ط��ӻ���������ɫ���ȶ�����
SO2�����л�ɫ�ط��ӻ���������ɫ���ȶ�����
����������֪X��YΪ�ǽ������ʣ�XΪ��ɫ��ĩ֤��ΪS��F��G��YΪ���壬D��һ���ᣬHΪ��ɫ��״�����ж�ΪCu��OH��2��IΪ����ɫ���壮A��D��I��Һ�μ�BaCl2��Һ��������ɫ������DΪ�����������Ʒ�Ӧ���ж�DΪH2SO4��B����ɫ��Ӧ�ʻ�ɫ˵��������Ԫ�أ����DΪ�ᣬB+D=X+F��X+Y=F����Ӧ��XΪS���ʣ��ƶ�BΪNa2S2O3��FΪSO2��YΪO2��ZΪ�����������ƶ�Ϊ
Cu��C+O2��Cu��Cu+O2=E���ж�EΪCuO��AΪCuSO4��F��G��YΪ���壬GΪNH3��IΪ����ɫ������������ͭ���ڰ�ˮ���ɵ�����IΪ��[Cu��NH3��4]SO4?H2O����ҵ�ϳ����÷�Ӧ������Z�����÷�Ӧ�ۢ��Ʊ�A��һ����C����Ӧ�ڢ���ȫ�õ�Eʱ���������䣮�ƶϳ�Z+Y=E��ӦΪ��2Cu+O2=2CuO��C�� ����ͭԪ�أ�������ͬ�ƶϳ�CΪCu2S�������ƶϳ������ʷ������
Cu��C+O2��Cu��Cu+O2=E���ж�EΪCuO��AΪCuSO4��F��G��YΪ���壬GΪNH3��IΪ����ɫ������������ͭ���ڰ�ˮ���ɵ�����IΪ��[Cu��NH3��4]SO4?H2O����ҵ�ϳ����÷�Ӧ������Z�����÷�Ӧ�ۢ��Ʊ�A��һ����C����Ӧ�ڢ���ȫ�õ�Eʱ���������䣮�ƶϳ�Z+Y=E��ӦΪ��2Cu+O2=2CuO��C�� ����ͭԪ�أ�������ͬ�ƶϳ�CΪCu2S�������ƶϳ������ʷ������
�����֪X��YΪ�ǽ������ʣ�XΪ��ɫ��ĩ֤��ΪS��F��G��YΪ���壬D��һ���ᣬHΪ��ɫ��״�����ж�ΪCu��OH��2��IΪ����ɫ���壮A��D��I��Һ�μ�BaCl2��Һ��������ɫ������DΪ�����������Ʒ�Ӧ���ж�DΪH2SO4��B����ɫ��Ӧ�ʻ�ɫ˵��������Ԫ�أ����DΪ�ᣬB+D=X+F��X+Y=F����Ӧ��XΪS���ʣ��ƶ�BΪNa2S2O3��FΪSO2��YΪO2��ZΪ�����������ƶ�ΪCu��C+O2��Cu��Cu+O2=E���ж�EΪCuO��AΪCuSO4��F��G��YΪ���壬GΪNH3��IΪ����ɫ������������ͭ���ڰ�ˮ���ɵ��������Ҵ��нᾧ����IΪ��[Cu��NH3��4]SO4?H2O����ҵ�ϳ����÷�Ӧ������Z�����÷�Ӧ�ۢ��Ʊ�A��һ����C����Ӧ�ڢ���ȫ�õ�Eʱ���������䣮�ƶϳ�Z+Y=E��ӦΪ��2Cu+O2=2CuO��C�к���ͭԪ�أ�������ͬ��Cu2S��2CuOĦ��������ͬ���ƶϳ�CΪCu2S��
��1�������ƶϿ�֪C�Ļ�ѧʽΪ��Cu2S�� I����Ļ�ѧʽΪ��[Cu��NH3��4]SO4?H2O��
�ʴ�Ϊ��Cu2S��[Cu��NH3��4]SO4?H2O��
��2��GΪNH3���õ���ʽ��ʾ���γɹ���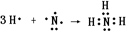 ��
��
�ʴ�Ϊ��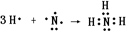 ��
��
��3���ö��Ե缫���AΪCuSO4��Һ���������������ӷŵ���������������ͭ���ӵõ���������ͭ����Ӧ�����ӷ���ʽΪ��2Cu2++2H2O
2 Cu+O2��+4H+����Ӧ�����������������Ʒ�Ӧ�������ʣ����������ˮ����Ӧ�����ӷ���ʽΪ��S2O32-+2H+�TS��+SO2��+H2O��
�ʴ�Ϊ��2Cu2++2H2O
2 Cu+O2��+4H+��S2O32-+2H+�TS��+SO2��+H2O��
��4��F������SO2����Ư���ԣ���Ư��ԭ��Ϊ��SO2�����л�ɫ�ط��ӻ���������ɫ���ȶ����ʣ�
�ʴ�Ϊ��SO2�����л�ɫ�ط��ӻ���������ɫ���ȶ�����
��1�������ƶϿ�֪C�Ļ�ѧʽΪ��Cu2S�� I����Ļ�ѧʽΪ��[Cu��NH3��4]SO4?H2O��
�ʴ�Ϊ��Cu2S��[Cu��NH3��4]SO4?H2O��
��2��GΪNH3���õ���ʽ��ʾ���γɹ���
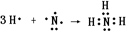 ��
���ʴ�Ϊ��
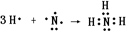 ��
�� ��3���ö��Ե缫���AΪCuSO4��Һ���������������ӷŵ���������������ͭ���ӵõ���������ͭ����Ӧ�����ӷ���ʽΪ��2Cu2++2H2O
| ||
�ʴ�Ϊ��2Cu2++2H2O
| ||
��4��F������SO2����Ư���ԣ���Ư��ԭ��Ϊ��SO2�����л�ɫ�ط��ӻ���������ɫ���ȶ����ʣ�
�ʴ�Ϊ��SO2�����л�ɫ�ط��ӻ���������ɫ���ȶ�����
���������⿼��������ת����ϵ���������ʵ��ۺ�Ӧ�ã���Ҫ��ͭ���仯�������ʵ�Ӧ�ã�����ʽ��д������������ӷ���ʽ����Ӧ���ӷ���ʽ��д��������������ķ���Ӧ�ú�ת����ϵ�ǽ���ؼ�����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ