��Ŀ����
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λ��ͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ����֮���γɵ�������Ϊ 10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣨Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�� ���ʾ����
(1)(XY)2����������ԭ������㶼����8���ӽṹ����д����ṹʽ��_________________��(XY)2����W2��������WXY����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ���(KXY)��Һ��ʹ��̪��Һ�Ժ�ɫ���������ӷ���ʽ��ʾ��ԭ��____________________��
(2)��֪��Ȳ��һ�������¿����������ɱ���3CH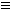 CH
CH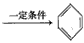 �������谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��_________________��
�������谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��_________________��
(3)�����谷��ǿ���ǿ����ˮ��Һ����ˮ�⣬�������ǻ�ȡ�������������ᡣ�����谷���ں����� �߶���һЩ�̷۳��ҷǷ������̷�����������Ʒ�ĺ��������׳Ƶ����������о����������谷���� ����������ϸ���н�ϳ����Ӷ��γ�����ʯ��������С�ܣ����������˥�ߣ�������Σ����������д�� �����谷��ϡ���ᷴӦֱ������������������ӷ���ʽ��_______________________��
(4)��̼�������Ե��ܺġ�����Ⱦ�����ŷ�Ϊ�����ľ���ģʽ������һ�ּ����ǽ�XZ2ת�����л���ʵ��̼ѭ�����磺
2XZ2(g)+2W2Z(l) =X2W4(g)+ 3Z2(g) ��H=+1411.0 kJ/mol
2XZ2(g)+3W2Z(l) =X2W5ZW(1)+3Z2(g) ��H=+1366.8 kJ/mol
����X2W4ˮ����X2W5ZW��Ӧ���Ȼ�ѧ����ʽΪ_____________________��
(5)����X2W5ZWȼ�ϵ�������ͼ��ʾ��װ�ã�
(1)(XY)2����������ԭ������㶼����8���ӽṹ����д����ṹʽ��_________________��(XY)2����W2��������WXY����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ���(KXY)��Һ��ʹ��̪��Һ�Ժ�ɫ���������ӷ���ʽ��ʾ��ԭ��____________________��
(2)��֪��Ȳ��һ�������¿����������ɱ���3CH
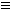 CH
CH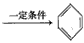 �������谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��_________________��
�������谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�Ľṹ��ʽ��_________________�� (3)�����谷��ǿ���ǿ����ˮ��Һ����ˮ�⣬�������ǻ�ȡ�������������ᡣ�����谷���ں����� �߶���һЩ�̷۳��ҷǷ������̷�����������Ʒ�ĺ��������׳Ƶ����������о����������谷���� ����������ϸ���н�ϳ����Ӷ��γ�����ʯ��������С�ܣ����������˥�ߣ�������Σ����������д�� �����谷��ϡ���ᷴӦֱ������������������ӷ���ʽ��_______________________��
(4)��̼�������Ե��ܺġ�����Ⱦ�����ŷ�Ϊ�����ľ���ģʽ������һ�ּ����ǽ�XZ2ת�����л���ʵ��̼ѭ�����磺
2XZ2(g)+2W2Z(l) =X2W4(g)+ 3Z2(g) ��H=+1411.0 kJ/mol
2XZ2(g)+3W2Z(l) =X2W5ZW(1)+3Z2(g) ��H=+1366.8 kJ/mol
����X2W4ˮ����X2W5ZW��Ӧ���Ȼ�ѧ����ʽΪ_____________________��
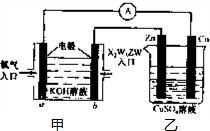
(5)����X2W5ZWȼ�ϵ�������ͼ��ʾ��װ�ã�
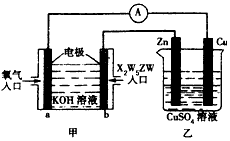
�ٸ�װ����Cu��Ϊ______����
��д��b���ĵ缫��Ӧʽ��_____________��
�۵�ͭƬ�������仯12.8 gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ_________L���ס�����װ���е� ������ҺpH�ı仯Ϊ��______����_______����������С�����䡱����
��д��b���ĵ缫��Ӧʽ��_____________��
�۵�ͭƬ�������仯12.8 gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ_________L���ס�����װ���е� ������ҺpH�ı仯Ϊ��______����_______����������С�����䡱����
(1)N C-C
C-C N��CN-+H2O
N��CN-+H2O HCN+OH-
HCN+OH-
 C-C
C-C N��CN-+H2O
N��CN-+H2O HCN+OH-
HCN+OH- (2)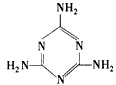
(3)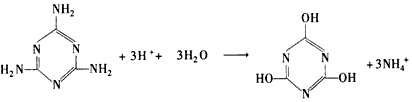

(3)
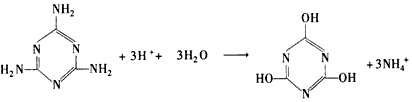
(4)C2H4(g)+H2O(l)=C2H5OH(l) ��H=-44. 2 kJ/mol
(5)��������C2H5OH+16OH--12e-=2CO32-+11H2O����2.24����������
(5)��������C2H5OH+16OH--12e-=2CO32-+11H2O����2.24����������

��ϰ��ϵ�д�
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
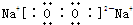

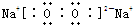
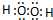
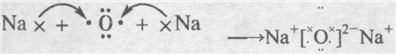

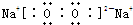
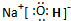
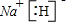
 ��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��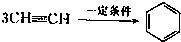 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��