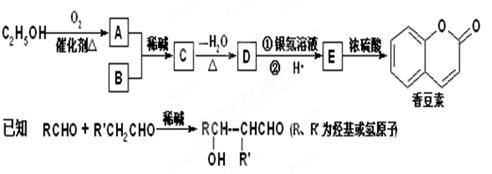
��Ŀ����
�Ҵ���һ����Ҫ�Ļ���ԭ�ϡ�
(1)�������ھƻ�ø��������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��__________________��
(2)���³�ѹ�£�23g�Ҵ���ȫȼ�����ɶ�����̼��ˮʱ����680kJ�����ʾ�÷�Ӧ���Ȼ�ѧ����ʽ��
________________________��
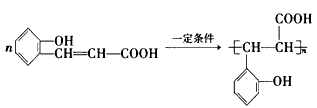
(3)�㶹����һ����;�㷺�����ϣ������������Ҵ���Bͨ������;���ϳɣ�����B�ķ���ʽΪC7H6O2��
(1)�������ھƻ�ø��������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��__________________��
(2)���³�ѹ�£�23g�Ҵ���ȫȼ�����ɶ�����̼��ˮʱ����680kJ�����ʾ�÷�Ӧ���Ȼ�ѧ����ʽ��
________________________��
(3)�㶹����һ����;�㷺�����ϣ������������Ҵ���Bͨ������;���ϳɣ�����B�ķ���ʽΪC7H6O2��

��֪��RCHO+ R'CH2CHO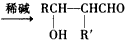 ��R��R'Ϊ��������ԭ�ӣ�
��R��R'Ϊ��������ԭ�ӣ�
��D�Ľṹ��ʽ��____________________��
��д�����л�ѧ����ʽE��һ�������·����ӳɾۺϷ�Ӧ��___________________________��
E���㶹�أ�_______________________��
��B�ж���ͬ���칹�壬���б�����ֻ��һ������������ͬ���칹��Ľṹ��ʽ��________________��
���й��㶹�ص�˵����ȷ����_________������ĸ����
a��ֻ�����嵥�ʷ����ӳɷ�Ӧ�����ܷ���ȡ����Ӧ
b��1 mol�㶹�ؿ���5 mol H2�����ӳɷ�Ӧ
c��1 mol�㶹�ؿ���2 mol NaOH������Ӧ
d��1 mol�㶹����ȫȼ������9.5 mol O2
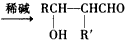 ��R��R'Ϊ��������ԭ�ӣ�
��R��R'Ϊ��������ԭ�ӣ���D�Ľṹ��ʽ��____________________��
��д�����л�ѧ����ʽE��һ�������·����ӳɾۺϷ�Ӧ��___________________________��
E���㶹�أ�_______________________��
��B�ж���ͬ���칹�壬���б�����ֻ��һ������������ͬ���칹��Ľṹ��ʽ��________________��
���й��㶹�ص�˵����ȷ����_________������ĸ����
a��ֻ�����嵥�ʷ����ӳɷ�Ӧ�����ܷ���ȡ����Ӧ
b��1 mol�㶹�ؿ���5 mol H2�����ӳɷ�Ӧ
c��1 mol�㶹�ؿ���2 mol NaOH������Ӧ
d��1 mol�㶹����ȫȼ������9.5 mol O2
(1)C6H12O6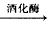 2C2H5OH+2CO2��
2C2H5OH+2CO2��
(2)C2H5OH(1)+3O2(g)=2CO2(g)+3H2O(1) ��H=-1360 kJ��mol-1
 2C2H5OH+2CO2��
2C2H5OH+2CO2�� (2)C2H5OH(1)+3O2(g)=2CO2(g)+3H2O(1) ��H=-1360 kJ��mol-1
(3)�� ��
��
�� ��
��
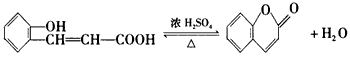
��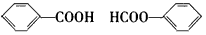 ��
��
��cd��
 ��
����
 ��
��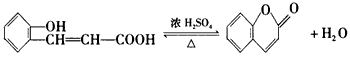
��
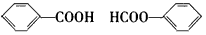 ��
����cd��

��ϰ��ϵ�д�
�����Ŀ

 ��R��R?������
��R��R?������