��Ŀ����
��13�֣���ҵ�ϳɰ������Ṥҵ�е���Ҫ���裬��֪N2(g) +3H2(g)  2NH3(g) ��H����92.4kJ��mol��1����ش�
2NH3(g) ��H����92.4kJ��mol��1����ش�
(1) ���ϳɰ���Ӧ�ﵽƽ��ı�ijһ������������ı�N2�� H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��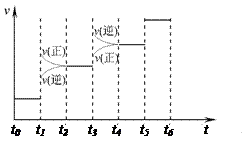
ͼ��t1ʱ����ƽ���ƶ������������� __ , t3ʱ����ƽ���ƶ������������� __ ___ , ���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ���� ��
(2) �¶�ΪT ��ʱ����2a mol H2��a mol N2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50������÷�Ӧ�Ļ�ѧƽ�ⳣ����ֵΪ ___________________�����ڴ������д����������������
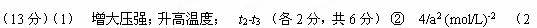
N2(g) + 3H2(g)  2NH3(g)
2NH3(g)
��ʼ���ʵ���Ũ��(mol.L-1) 2a 4a 0
���ʵ�Ũ�ȱ仯(mol.L-1) a 3a 2a
ƽ�����ʵ���Ũ��(mol.L-1) a a 2a ��3�֣� ��2�֣�
��2�֣�
����

��ϰ��ϵ�д�
�����Ŀ



 2NH3(g) ��H��0�� 2SO2(g)+O2(g)
2NH3(g) ��H��0�� 2SO2(g)+O2(g)
 2NH3(g) ��H����92.4kJ��mol��1����ش�
2NH3(g) ��H����92.4kJ��mol��1����ش�