��Ŀ����
��ʵ����������һ�����ʵ���Ũ����Һ���йز����У���ȷ����
- A.����0.1mol��L-1NaOH��Һ�����У���NaOH���������ֽ�ϳ���
- B.����0.1mol��L-1��H2SO4��Һʱ������ȡ��ŨH2SO4��������ƿ�м�ˮϡ��
- C.����0.1mol��L-1��NaCl��Һʱ������ý�ͷ�ιܼ�ˮ���̶���
- D.����0.1mol��L-1��HCl��Һʱ��Ҫ����ȡŨ�������Ͳ���ܽ��õ��ձ�ϴ��2��3�Σ�����ϴ��Һת������ƿ��
B

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
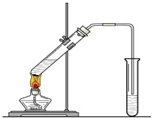 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺
 ��Ӧʱ���������ɵ������ǣ� ��
��Ӧʱ���������ɵ������ǣ� ��