题目内容
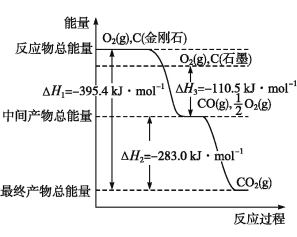
【题目】某校化学兴趣小组设计了图示实验装置(图中省略了夹持仪器)来测定某铁碳合金中铁的质量分数。

(1)mg铁碳合金中加入过量浓硫酸,未点燃酒精灯前,A、B均无明显现象,其原因是:
① 常温下碳与浓硫酸不反应;②________________________。
(2)写出加热时A中碳与浓硫酸发生反应的化学方程____________________________。
(3)B中的现象是:_______________________;C的作用是:___________________。
(4)待A中不再逸出气体时,停止加热,拆下E并称重,E增重bg.则铁碳合金中铁的质量分数为________________(写表达式)。
(5)甲同学认为利用此装置测得铁的质量分数偏大,请你写出可能的原因:_____________________。
【答案】 常温下Fe遇浓硫酸发生钝化 C+2H2SO4(浓)![]() CO2↑+2SO2↑+2H2O 品红溶液褪色 除尽反应产物中的SO2气体
CO2↑+2SO2↑+2H2O 品红溶液褪色 除尽反应产物中的SO2气体 ![]() ×100% 装置内的CO2难以赶尽,导致E质量增重偏小
×100% 装置内的CO2难以赶尽,导致E质量增重偏小
【解析】(1)常温下,铁与浓硫酸反应生成一层致密的氧化物保护膜,阻止内金属继续与浓硫酸反应,发生钝化现象,故答案为:常温下Fe遇浓硫酸发生钝化;
(2)碳与浓硫酸在加热的条件下生成二氧化硫、二氧化碳、水,反应方程式为C+2H2SO4(浓)![]() CO2↑+2SO2↑+2H2O,故答案为:C+2H2SO4(浓)
CO2↑+2SO2↑+2H2O,故答案为:C+2H2SO4(浓)![]() CO2↑+2SO2↑+2H2O;
CO2↑+2SO2↑+2H2O;
(3)A中生成的气体含有CO2、SO2,SO2具有漂白性,能使品红溶液褪色;C装置的作用为除尽反应产物中的SO2气体,防止影响二氧化碳的质量测定,故答案为:品红溶液褪色;除尽反应产物中的SO2气体;
(4)E增重b g为二氧化碳的质量,根据碳元素守恒可知合金中碳元素的质量为![]() bg,合金中铁的质量为mg-
bg,合金中铁的质量为mg-![]() bg=(m-
bg=(m-![]() b)g,故铁的质量分数
b)g,故铁的质量分数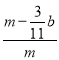 =
=![]() ,故答案为:
,故答案为: ![]() ;
;
(5)装置内残留部分二氧化碳,未能被装置E完全吸收,导致测量的二氧化碳的质量偏小,即合金中碳元素的质量偏小,铁元素的质量增大,质量分数增大,故答案为:装置内的CO2难以赶尽,导致E质量增重偏小。

【题目】关于强弱电解质及非电解质的组合完全正确的是( )
A | B | C | D | |
强电解质 | NaCl | H2SO4 | CaCO3 | HNO3 |
弱电解质 | HF | BaSO4 | HClO | CH3COOH |
非电解质 | Cl2 | CO2 | H2S | SO2 |
A.AB.BC.CD.D