��Ŀ����
��10����1L�ĺ����ܱ�������ͨ��a mol����A��a mol����B����һ�������·�����Ӧ��xA(g)��yB(g)  pC(g)��qD(g)
pC(g)��qD(g)
��֪��ƽ����Ӧ����v(C)��0.5v(A)����Ӧ2 minʱ�ﵽƽ�⣬A��Ũ�ȼ�����һ�룬B�����ʵ���������0.5amol����0.75a mol D���ɡ��ش��������⣺
(1)��Ӧ2 min�ڣ�vA��________________��
(2)��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
(3)��Ӧ��ƽ��ʱ��B��ת����Ϊ________��
(4)������˵���÷�Ӧ����ƽ�����________________��
A.�����ڵ���ѹǿ���ٱ仯
B.�����������ƽ����Է����������ٱ仯
C.������������ܶȲ��ٱ仯
D.������D���������ٱ仯
(5)����÷�Ӧ�Ļ�ѧƽ�ⳣ��________________��
 pC(g)��qD(g)
pC(g)��qD(g)��֪��ƽ����Ӧ����v(C)��0.5v(A)����Ӧ2 minʱ�ﵽƽ�⣬A��Ũ�ȼ�����һ�룬B�����ʵ���������0.5amol����0.75a mol D���ɡ��ش��������⣺
(1)��Ӧ2 min�ڣ�vA��________________��
(2)��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
(3)��Ӧ��ƽ��ʱ��B��ת����Ϊ________��
(4)������˵���÷�Ӧ����ƽ�����________________��
A.�����ڵ���ѹǿ���ٱ仯
B.�����������ƽ����Է����������ٱ仯
C.������������ܶȲ��ٱ仯
D.������D���������ٱ仯
(5)����÷�Ӧ�Ļ�ѧƽ�ⳣ��________________��
��0.25amol/(L��min) ��2A+2B="C+3D" ��50�� ��D ��27/16
�����������1��A��Ũ�ȼ�����һ�룬vA��0.5amol¸1L ¸2 min=0.25amol/(L��min)��
��2��ƽ����Ӧ����v(C)��0.5v(A)������x:p=2:1��
xA(g) �� yB(g)
 pC(g) �� qD(g)
pC(g) �� qD(g)��ʼ��amol/L amol/L 0 0
�仯��0.5amol/L 0.5amol/L 0.25amol/L 0.75amol/L
ƽ�⣺0.5amol/L 0.5amol/L 0.25amol/L 0.75amol/L
X:y:p:q=2:2:1:3
�÷�Ӧ�Ļ�ѧ����ʽ��2A(g)��2B(g)
 C(g)��3D(g)
C(g)��3D(g)(3) B��ת����=0.5amol/L¸ amol/L=0.5����50��
(4) ��Ӧ����ƽ��ʱ�������ʵ�Ũ�ȣ����������ֲ��䡣���ڷ�Ӧ��ǰ���������������ں����ܱ���������������ڵ���ѹǿ�������������ƽ����Է���������������������ܶȾ�������Ϊƽ����־����ѡD��
��5����ѧƽ�ⳣ������ƽ��ʱ�������뷴Ӧ���Ũ����֮�ȣ���Ӧ�Ļ�ѧƽ�ⳣ��=0.25a´(0.75a )3¸(0.5a)2´(0.5a)2=27/16
��������Ϊ�йػ�ѧƽ��ĸ�����⻯ѧƽ�⡰����ʽ������ģʽ��

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ
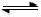 2NO(g) ��H > 0
2NO(g) ��H > 0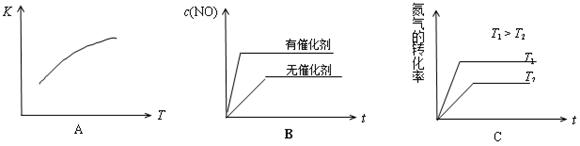
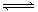 2AB3��g�� ��H<0������ͼ����ȷ���ǣ� ��
2AB3��g�� ��H<0������ͼ����ȷ���ǣ� ��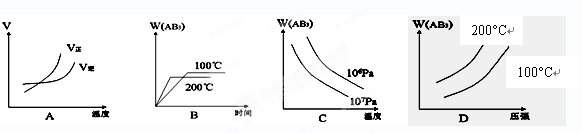
 cP(g)��dQ(g)�ﵽƽ��ʱ��M���������y(M)�뷴Ӧ�����Ĺ�ϵ����ͼ��ʾ������z��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȡ�����˵����ȷ����
cP(g)��dQ(g)�ﵽƽ��ʱ��M���������y(M)�뷴Ӧ�����Ĺ�ϵ����ͼ��ʾ������z��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȡ�����˵����ȷ���� 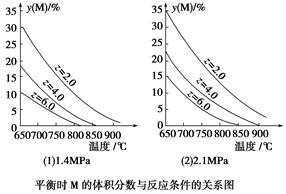
 2SO2(g)��O2(g) ��H��0������SO3�ı仯����ͼ��ʾ��
2SO2(g)��O2(g) ��H��0������SO3�ı仯����ͼ��ʾ��
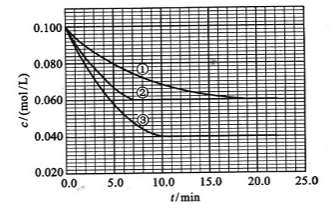
 C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ
C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ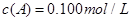 ��
��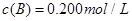 ��
��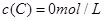 ����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��
����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��