��Ŀ����
����Ŀ���Ķ����챨�棬�ش��������⡣
����Ұ�⿼������������ʵ��������Ҫ;��֮һ������ij��ѧ������ȤС�����й���ɽ������ʹ�����Ұ�⿼�죬��д���챨�档

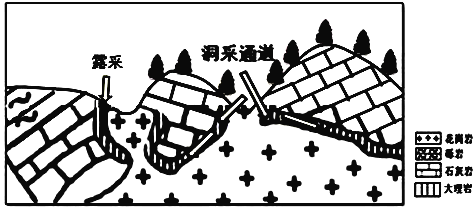
��1�������¼���Тٴ�����ܼ�¼����________��˫��ѡ���⣩��
A.������� B.�����㲼 C.����ƽ̹ D.�ر����
��2������¼����������Ŀ֮�⣬��ò���۵������ǶȻ���________��________�ȡ�
��3�����ݵ�ò�γ������жϣ��뿦˹�ص�ò�γɼ��ص�������ص���Ȼ������________��˫��ѡ���⣩��
A.ֲ�� B.���� C.��ʯ D.ˮ��
��4���Ķ����챨�棬�����˴ο�������Ҫ����
��5���ԡ���չѧϰ���ķ�����ͼ�еĵ�ò���з��࣬��˵�����ɡ�
���𰸡���1��AD
��2����ģ �ֲ����ʵء��ر�ֲ���ȣ�
��3��CD
��4���۲졢ʶ��������˹�ص�ò���ۼ�����Ҫ�ص㣬̽�������
��5�����ಢ˵�����ɣ��������ɡ����磺�����ࡣ���֡������Ϊһ�࣬������ˮ��ò����ʴĢ����ɳ��Ϊһ�࣬���ڷ�ɵ�ò��
��������
������Ҫ����ѧ����ȡ�ͽ����Ϣ�������������ס�
��1���ɿ��챨���֪���ٴ����ܵ�ò����˹�ص�ò���������ر���������������AD��ȷ��C����ͼ����Ϣδ���������������ҷ�ɽ����γ�ȡ�ɽ�庣�θ߶Ƚϵͣ�����ֽ��ޱ�����������B����
��2����ò���۵�����������Ҫ��������̬��������ɡ�ɫ�ʡ���ģ���ֲ���ֲ���ȡ�
��3����˹�ص��ε��γ���ʯ���ҵ�������ˮ������ʴ�Ľ����ʯ���ҵ���Ҫ�ɷ���̼��ƣ�CaCO3��������ˮ�Ͷ�����̼ʱ������ѧ��Ӧ����̼�����[Ca(HCO3)2]��̼���+������̼+ˮ��̼�����---CaCO3+CO2+H2O=Ca��HCO3��2�����߿�����ˮ���������ܶ��γɲ�����������뿦˹�ص�ò�γɼ��ص�������ص���Ȼ��������ʯ��ˮ�ģ���C��D��ȷ��
��4���������鱨���֪���˴ο�������Ҫ�����ǹ۲졢ʶ��������˹�ص�ò���ۼ�����Ҫ�ص㣬̽�������
��5���ķ�����ͼ�еĵ�ò��Ϊ�������ö��ɣ������ò����Ҫ���������У���ˮ���硢���˺ͱ������á�����Ϊ��ˮ��ѧ��ʴ���ɡ�����ȷ����ں�����ɽ�ڣ�����ˮ�������ɣ�����������ˮ��ò����ʴĢ���������ʴ���ɣ�ɳ��������ѻ����ɣ��������ڷ�ɵ�ò��

 ��У����ϵ�д�
��У����ϵ�д�