��Ŀ����
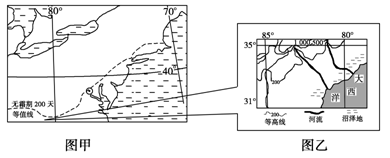
����һ ��ͼ�еļ�ͼΪ����ij��ũ�����ҵ���ֲ�ͼ����ͼΪ�ҹ�ijʡũ�����ҵ���ֲ�ͼ��

���϶� 2008�����Σ���ķ�����2011��ŷծΣ���ı�����ʹ��ͼ11�мס����������������������������������ƻ�����������Ʒ�۸���ǣ����ӵľ��û���ʹ�������Ĺ�ҵ�����൱���ѡ�
��1���ֱ�˵��ͼ�м�����ʡ���߱����߽�ĵ��ζԼ�����ʡ���÷�չ��ͻ����Ӱ�졣��8�֣�
��2��˵��ͼ����������١��ں���ʳ����ۡ��ܵ����ơ�������������������ʳ�����ı��ؾ����½��������ܡ��߲˵�ũ��Ʒ�ı���ȴ��������������Ҫ����ᾭ��ԭ��10�֣�
��1���������߽��ߴ��ĵ�����ɽ�أ�������˹ɽ��Ϊ������2�֣���������������½�Ͻ�ͨ��ϵ����2�֣���ʡ�������ߴ��ĵ�����ƽԭΪ������2�֣�����������ʡ�Ľ�ͨ��ϵ�;��ú�������2�֣���2��������魣���1�֣����Ͳˣ���1�֣������ף���С����1�֣���ˮ������1�֣���ᾭ��ԭ�ף���ͨ���������ĸ��ƺ�ũ��Ʒ���ʡ���صȼ����ķ�չ���ܷ���ؽ����ܡ��߲˵�ũ��Ʒ��Ӧ��ŷ�ޱ������Ӷ�ʹũҵ�����ڵ����ϴ�Ϊ��չ����3�֣��ң����ڻ�������ҵ���ͳ�����������Ⱥ�ķ�չ���Ի��ܡ��߲˵�ũ��Ʒ���г����������ӡ���3�֣�