��Ŀ����
����Ŀ����2017�¿α��������϶������Dzⶨ�������е������ľ��䷽������ԭ������Ũ�����ڴ��������½���Ʒ���л���ת������Σ�������ͼ��ʾװ�ô�����Σ�Ȼ��ͨ���ζ���������֪��
NH3+H3BO3=NH3��H3BO3��
NH3��H3BO3+HCl= NH4Cl+ H3BO3��
�ش��������⣺
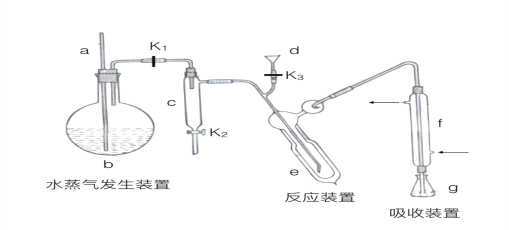
��1��a��������_______________��
��2��b�з����������Ƭ��Ŀ����____________��f��������__________________��
��3����ϴ������g�м�����ˮ����k1���ر�k2��k3������b������������·��ֹͣ���ȣ��ر�k1��g������ˮ��������c��ԭ����____________����k2�ŵ�ˮ���ظ�����2~3�Ρ�
��4��������ϴ��g�м������ᣨH3BO3����ָʾ�������������dע��e�����ע������������Һ��������ˮ��ϴd���ر�k3��d�б�������ˮ����k1������b��ʹˮ��������e��
��d�б�������ˮ��Ŀ����___________________��
��e����Ҫ��Ӧ�����ӷ���ʽΪ________________��e�����п�˫�㲣��ƿ��������________��
��5��ȡij�ʰ��ᣨC2H5NO2����Ʒm �˽��вⶨ���ζ�g������Һʱ����Ũ��Ϊcmol��L-1������V mL������Ʒ�е�����������Ϊ_________%����Ʒ�Ĵ��ȡ�_______%��
���𰸡���1������b��ѹǿ����
��2����ֹ���� ֱ��������
��3��c���¶��½�����·���γɸ�ѹ
��4����Һ�⣬��ֹ�����ݳ� ��NH4��+OH![]() NH3��+H2O ����ʹ����ȫ����
NH3��+H2O ����ʹ����ȫ����
��5��![]()
![]()
����������1��a�е������������������������ƽ����ѹ���Ա���b��ѹǿ������
��2��b�з����������Ƭ��Ŀ���Ƿ�ֹ���С�f��������ֱ�������ܡ�
��3������c��e���������ӵĹܵ���ˮ��������Ϊˮ����ѹԶС��������ѹ���ڴ���ѹ�������£������ƿ�ڵ�����ˮ��������c�С�
��4�������������壬���d�б�������ˮ��Ŀ����Һ�⣬��ֹ�����ݳ�����e����Ҫ��Ӧ���������ڼ��������µķ�Ӧ�����ӷ���ʽΪNH4++OH-![]() NH3��+H2O��e�����п�˫�㲣��ƿ�������DZ��¼���������ʧ��������笠�ת��Ϊ�����ݳ���
NH3��+H2O��e�����п�˫�㲣��ƿ�������DZ��¼���������ʧ��������笠�ת��Ϊ�����ݳ���
��5��ȡij�ʰ��ᣨC2H5NO2����Ʒm �˽��вⶨ���ζ�g������Һʱ����Ũ��Ϊc mol��L-1������V mL�����ݷ�ӦNH3��H3BO3+HCl��NH4Cl+H3BO3�����������Ʒ��n(N)��n(HCl)��c mol��L-1![]() ��0.001cV mol������Ʒ�е�����������Ϊ
��0.001cV mol������Ʒ�е�����������Ϊ![]() ����Ʒ�иʰ����������0.001cV
����Ʒ�иʰ����������0.001cV![]() ��������Ʒ�Ĵ��ȡ�
��������Ʒ�Ĵ��ȡ�![]() ��
��
����ʦ��������ȷ������ʵ����ʺ�ʵ��ԭ���ǽ��Ĺؼ���ע����������ۺ���ʵ�������������Ļ������̣�ԭ������Ӧ���ʡ�����װ�á���������ۡ�������������롣�������Ϊ����1��ʵ���Ǹ���ʲô���ʺ�ԭ����Ƶģ�ʵ���Ŀ����ʲô����2�����ø��������ơ�״̬��������(����ʵ��Ŀ�ĺ���صĻ�ѧ��Ӧԭ��������ȫ��ķ����ȽϺ�������������ѡ��)����3���й�װ�ã����ܡ�ʹ�÷��������÷�Χ��ע�����⡢�Ƿ������װ�ÿ��á��������ȡ���4���йز��������ܡ�����˳��ע��������������ĺ������5��ʵ���������¶��ϣ��������ȫ��۲졣��6��ʵ����ۣ�ֱ�ӽ��ۻ����ۡ��ڶ�ʵ�鷽�������Ҫ�㣺��1�����Ҫ�㣺ʵ�鷽�������Ҫ��ȷ����Ҫ�㣺����Ŀ��������Ҫ���������ҩƷ��װ�õ���������ע��ʵ������еİ�ȫ�Բ��������ử��ʵ��װ��ͼ����ע�������Ĺ����Ҫ������������ը����ȼ�ա������С�������������ˮ����������ȴ��ˮԡ����ʱ��ȡ��Ӧ��ʩ����ͬһ�����ڲ�ͬλ�õ���Ӧ���õȣ���Ҫ�ϸ��ա�����(ʵ�鲽��)�������ۡ��ĸ�ʽ������