��Ŀ����
| |||||||||||||||||||||||||||

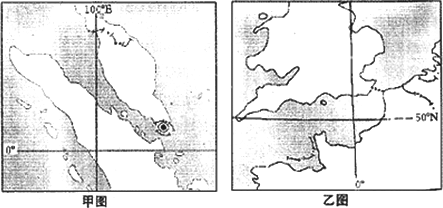
 53���ò�ϵ�д�
53���ò�ϵ�д�����ס�����ͼΪ����������ӵĵ���ʾ��ͼ����ͼ�ش�
��1����ͼ��ʾ���� �ӣ���ͼ��ʾ���� �ӡ�
��2�������Ұ����ܺ�ˮ��ʴ���� ͼ��ʾ�������������������ԭ����Ҫ�� ��
��3�� ͼ��ʾ������������Խ�С����Ϊ�úӴ����� ��������������ˮ��Դ����Ӧ������������������ԭ�����������Խ�С�⣬������ ������һͼ��ʾ�����������������ڱ仯 ����Ϊ�úӴ����� �����������ﵭˮ��Դ��Ȼ�ḻ���������˿� �����ٱ����á�
��4����ͼ��A��ˮ�����̵������� ���ù��̴�����һϵ�о���Ч�棬ͬʱҲ������ ����̬���⣻����ͼ��ʾ�������������� ��������̬������
��5��Ŀǰ��������������Ĺ��ҹ�ͬ���ٵ���ᷢչ�����У� ��
| A���Ͷ������� | B��������ҵ�����д���� |
| C������������� | D����������ҵѹ���� |