��Ŀ����
����Ŀ����2016���㽭�߿���ѡ�����Բ���A��������Ԫ����ɵĻ����ij�о�С�鰴��������̽������ɣ�
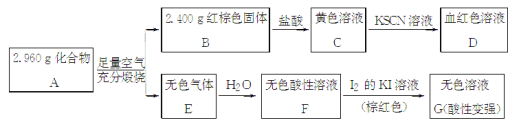
��ش�
(1)A�����Ԫ��Ϊ________(��Ԫ�ط��ű�ʾ)����ѧʽΪ________��
(2)��ҺC���ܽ�ͭƬ���оٸ÷�Ӧ��һ��ʵ��Ӧ��________________��
(3)��֪������A����ϡ���ᷴӦ������һ�ֵ���ɫ�������һ������(��״���µ��ܶ�Ϊ1.518 g��L��1)����������ӵĵ���ʽΪ________��д���÷�Ӧ�����ӷ���ʽ_____________________________________��
(4)д��F��G��Ӧ�Ļ�ѧ����ʽ________________________�����ʵ�鷽��̽����ҺG�е���Ҫ��(������H2O��H����K����I��)________��
������ (1)S��Fe Fe3S4 (2)��ӡˢ��·��
(3) ![]() Fe3S4��6H��===3H2S����3Fe2����S
Fe3S4��6H��===3H2S����3Fe2����S
(4)H2SO3��I2��H2O===H2SO4��2HI
ȡ��ҺG���������BaCl2��Һ����������ɫ����������SO![]() �����˺�ȡ��Һ���μ�H2O2��Һ�����ٲ�����ɫ����������H2SO3
�����˺�ȡ��Һ���μ�H2O2��Һ�����ٲ�����ɫ����������H2SO3
�������� (1)������A�����������г�����գ��õ�����ɫ����B����ɫ����E��B��������õ���ɫ��ҺC��C����KSCN��Һ�õ�Ѫ��ɫ��ҺD��˵��A�к���FeԪ�أ�BΪFe2O3��CΪFeCl3��Һ��DΪFe(SCN)3��Һ����ɫ����E����ˮ�õ���ɫ������ҺF��F�����غ�ɫI2��KI��Һ���õ���ɫ��ҺG������Һ�����Ա�ǿ���ݴ���֪EΪSO2��FΪH2SO3��GΪH2SO4��HI�Ļ��Һ����˻�����A�к���Fe��SԪ�ء�2.400 g Fe2O3�к���FeԪ�ص�����Ϊ2.400 g��![]() ��1.680 g����A��SԪ�ص�����Ϊ2.960 g��1.680 g��1.280 g����ô������A��Fe��Sԭ�Ӹ���֮��Ϊ
��1.680 g����A��SԪ�ص�����Ϊ2.960 g��1.680 g��1.280 g����ô������A��Fe��Sԭ�Ӹ���֮��Ϊ![]() ��
��![]() ��3��4����A�Ļ�ѧʽΪFe3S4��
��3��4����A�Ļ�ѧʽΪFe3S4��
(2)��ҺC�к���FeCl3�����ܽ�ͭƬ�������ķ�ӦΪ2FeCl3��Cu===2FeCl2��CuCl2���÷�Ӧ��������ӡˢ��·�塣
(3)A(Fe3S4)����ϡ���ᷴӦ������һ�ֵ���ɫ������(����S)��һ�����壬�������ڱ�״���µ��ܶ�Ϊ1.518 g��L��1���������Ħ������Ϊ1.518 g��L��1��22.4 L��mol��1��34.00 g��mol��1���Ӷ���֪������ΪH2S�������ʽΪ![]() �������֪��Ϣ���غ��ϵ���ɿ�֪���÷�Ӧ�����ӷ���ʽΪFe3S4��6H��===3H2S����3Fe2����S��
�������֪��Ϣ���غ��ϵ���ɿ�֪���÷�Ӧ�����ӷ���ʽΪFe3S4��6H��===3H2S����3Fe2����S��
(4)��ɫ������ҺF(H2SO3)�����غ�ɫI2��KI��Һ���õ���ɫ��ҺG(��H2SO4)����ѧ����ʽΪH2SO3��I2��H2O===H2SO4��2HI����ҺG����Ҫ����SO![]() ��H2SO3����������HCl������ǿ��H2SO3����̽����ҺG�е���Ҫ�������ȼ������BaCl2��Һ����������ɫ����������SO
��H2SO3����������HCl������ǿ��H2SO3����̽����ҺG�е���Ҫ�������ȼ������BaCl2��Һ����������ɫ����������SO![]() �����˺�����Һ�еμ�H2O2��Һ�����ٲ�����ɫ����������H2SO3��
�����˺�����Һ�еμ�H2O2��Һ�����ٲ�����ɫ����������H2SO3��

 һ����������ϵ�д�
һ����������ϵ�д�����Ŀ�����ӱ�ʡ��ɽ��2017�������ѧ�ڵ�����ģ�⿼�����ۻ�ѧ���⡿������ͼװ���Ʊ����������ռ��±��е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е���
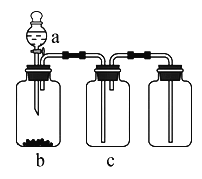
ѡ�� | ���� | a | b | c |
A | NO2 | Ũ���� | ͭƬ | NaOH��Һ |
B | H2 | ϡ���� | пƬ | Ũ���� |
C | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
D | O2 | ˫��ˮ | MnO2��ĩ | Ũ���� |