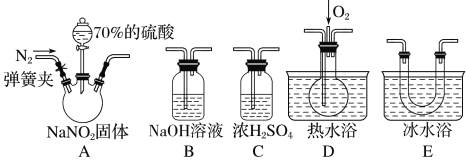
��Ŀ����
����Ŀ��A��F����Ԫ���У���F��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
Ԫ�� | ԭ�ӽṹ������ |
A | ���γɵ�һ��ͬλ��ԭ���ڿ����п��Ʋ⻯ʯ����� |
B | ��Aͬ���ڣ�������������ˮ��������Ũ��ϡ��Һ����ǿ������ |
C | �ؿ��к�������Ԫ�� |
D | Dԭ�ӵ��ڲ��������������������5�� |
E | �䵥����Ҫ�����ڻ�ɽ�ڸ��� |
F | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
��ش��������⣺
��1��A��Ԫ�����ڱ��е�λ����_______________��A��C�γɵĻ�����AC2�ĵ���ʽ��__________��
��2�� ������ʵ��֤��C��E�ķǽ�����ǿ������ ��(�����)
�ٳ����£�C�ĵ��ʳ���̬��E�ĵ��ʳʹ�̬
��C����̬�⻯����ȶ���ǿ��E����̬�⻯����ȶ���
��C��E�γɵĻ������У�E������
��E���⻯��ķе����C���⻯��ķе�
��3�� ��A��B��C����Ԫ������Ԫ�����γɵ�������������Ŀ֮��Ϊ1��1�����ӻ������� ���ѧʽ������������NaOH��Һ�ڼ���ʱ��Ӧ�����ӷ���Ϊ�� ��
��4�� FC������B������������ˮ�����ϡ��Һ��Ӧ�����ӷ���ʽ �����б�״����5.6L��BC���ɣ���ת�Ƶĵ�����Ϊ ��
��5�� A���⻯���ж��֣�1 mol A��ij���⻯������к���14 mol���ӣ���֪��25�桢101kPa�£�1g���⻯����������������ȫȼ������Һ̬ˮʱ�ų�������Ϊ40 kJ��д����ʾ���⻯��ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
���𰸡���1���ڶ����ڵ�IVA����![]() ����2���ڢۣ�
����2���ڢۣ�
��3��NH4HCO3��NH4++HCO3��+2OH��=CO32��+NH3��+2H2O��
��4��3FeO+10H++NO3��=3Fe3++NO��+5H2O��0.75NA��
��5��C2H2(g)��5/2O2(g)��2CO2(g)��H2O(l)��H����1040kJ��mol-1��
��������������Ϣ���Ƴ�A��C��BΪN��CΪO��D��Mg��EΪS��FΪFe����1��̼λ�ڵڶ����ڵ�IVA�壬�γɻ�������CO2�������ʽΪ��![]() ����2���ٷǽ������ǻ�ѧ���ʣ����ʵ�״̬���������ʣ�����֮��û�б�Ȼ��ϵ���ʴ���ͬ������ϵ��·ǽ����Լ��������⻯����ȶ��Լ�������֮Ҳ����������ȷ�����γɵĻ�������SO2��S�����ۣ�˵��S�ķǽ����Ա�O��������ȷ���ܷе�ĸߵ���ǽ�����ǿ���أ��ʴ���3���γɵĻ������������ӻ�����������Σ���������֮��Ϊ1��1����˻�������NH4HCO3�����������Ƽ��ȷ�Ӧ�����ӷ�Ӧ����ʽΪ��NH4����HCO3����2OH��
����2���ٷǽ������ǻ�ѧ���ʣ����ʵ�״̬���������ʣ�����֮��û�б�Ȼ��ϵ���ʴ���ͬ������ϵ��·ǽ����Լ��������⻯����ȶ��Լ�������֮Ҳ����������ȷ�����γɵĻ�������SO2��S�����ۣ�˵��S�ķǽ����Ա�O��������ȷ���ܷе�ĸߵ���ǽ�����ǿ���أ��ʴ���3���γɵĻ������������ӻ�����������Σ���������֮��Ϊ1��1����˻�������NH4HCO3�����������Ƽ��ȷ�Ӧ�����ӷ�Ӧ����ʽΪ��NH4����HCO3����2OH��![]() NH3����H2O��CO32������4��FC��FeO��B������������Ӧˮ������HNO3���������ǿ������,�ѣ�2��Fe������Fe3����������ӷ�Ӧ����ʽΪ3FeO��10H����NO3��=3Fe3����NO����5H2O��ת�Ƶ������ʵ���Ϊ5.6��3/22.4mol=0.75mol��������Ϊ0.75NA����5��1molA���⻯����14mol���ӣ�AΪC2H2��ȼ������1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų������������ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽΪ��C2H2(g)��5/2O2(g)��2CO2(g)��H2O(l) ��H=��26��40/1kJ��mol��1=��1040 kJ��mol-1��
NH3����H2O��CO32������4��FC��FeO��B������������Ӧˮ������HNO3���������ǿ������,�ѣ�2��Fe������Fe3����������ӷ�Ӧ����ʽΪ3FeO��10H����NO3��=3Fe3����NO����5H2O��ת�Ƶ������ʵ���Ϊ5.6��3/22.4mol=0.75mol��������Ϊ0.75NA����5��1molA���⻯����14mol���ӣ�AΪC2H2��ȼ������1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų������������ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽΪ��C2H2(g)��5/2O2(g)��2CO2(g)��H2O(l) ��H=��26��40/1kJ��mol��1=��1040 kJ��mol-1��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�