��Ŀ����
���±���ͼ���ҹ�����������AΪ������BΪ���ϣ�CΪ���������ޣ�������������λ�ֲ�ʾ��ͼ������������⣨13�֣�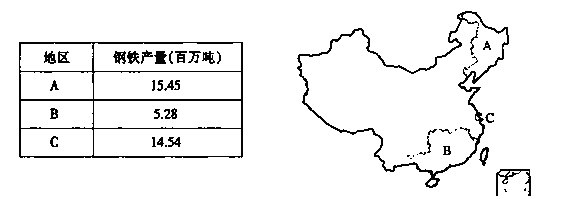
��1������A��B�����������������������Ҫ��Ȼ������2�֣���
��2��C�������ҹ����л�ˮƽ�ϸߵĵ���֮һ����Ҳ�����˽�Ϊ���صij��л����⣬
��Ҫ��������Щ���棿��3�֣�
��3��˵��A��������ƽԭ������C������������Ҫ��ũҵ�������ͣ�������A���������γɵ���λ��������Ҫ�����ص㡣
 �������ͣ�A ��C ����2�֣�
�������ͣ�A ��C ����2�֣�
A��λ���أ�4�֣���
A�����ص㣨2�֣���
��1��A:����ú̿��Դ�ḻ ��1�֣� B: ����ú̿��Դ�٣�1�֣�
��2�����������½�����ͨӵ������ס�������ҵ���ѡ���3�֣�
��3��A����������Ʒ����ũҵ��1�֣� C�أ�ˮ����ֲҵ��1�֣�
��Ȼ���أ��ļ����¶��꣬����ͬ�ڣ�����ƽ̹�����������������������֣�ˮԴ���㡣��2�֣�
��ᾭ�����أ��ع���ϡ��ũ��Ʒ��Ʒ�ʸߣ���е��ˮƽ�ߣ���ͨ�������г���������ҵ�ȽϷ�������������ֵȡ���2�֣� ����д�ĵ㼴�ɡ�
�����ص�:������ģ��е��ˮƽ�ߡ���2�֣�
�������������
��1��AΪ������BΪ���ϡ������������������������Ҫ��Ȼ����Ӧ�����ص���Ȼ��Դ���������з�������������ú����Դ�ḻ�����ϵ���ú����Դȱ����
��2�����л�������������Ҫ�л��������½�����ͨӵ������ס�������ҵ���ѵȡ�
��3��������������Ʒ����ũҵ�������ǵ�����ˮ����ֲҵ��������Ʒ����ũҵ�ķ�չӦ����Ȼ���ء���ᾭ��������������з�������Ʒ����ũҵ���ص����������ģ��е��ˮƽ�ߡ�
���㣺���⿼���ҹ�����������졣
�����������Ѷ�һ�㣬���ҹ����������ϡ�������������������������λ�ֲ�ʾ��ͼΪ�����������ظ���������ũҵ���������л������⡣ѧ�����������ҹ���������ĵ�������������������

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�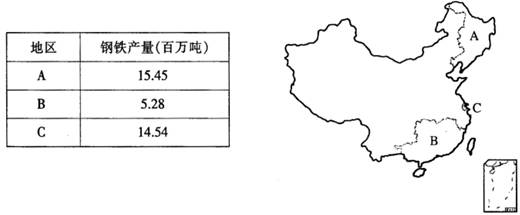
 ��1��A���������������������ܽӽ�������λѡ���Ƿ���ͬ����4�֣���
��1��A���������������������ܽӽ�������λѡ���Ƿ���ͬ����4�֣���
