��Ŀ����
���ݲ��Ϻ�ͼ���������ѧ֪ʶ���ش��������⡣
ϣ����ѧ����ʵ��С���ij�����ĸ�סլС���Ļ������й۲���㣬�õ��˰���(����)6��00��18��00���������ݲ�������ͼ20��ͼ21(���¶�ȡij��ƽ���¶�)��
����һ�������µ���������ʡ�
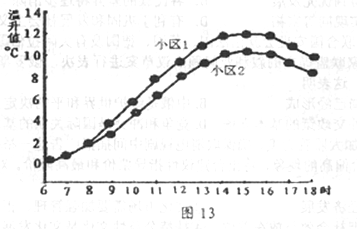
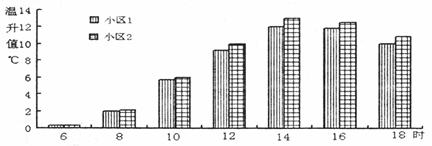
 ���϶���ͼ20��С����Ϊ�µ��������ʽϸߵ�С����ƽ��������Ϊ0.74��С����Ϊ�µ��������ʽϵ͵�С����ƽ��������Ϊ0.64��
���϶���ͼ20��С����Ϊ�µ��������ʽϸߵ�С����ƽ��������Ϊ0.74��С����Ϊ�µ��������ʽϵ͵�С����ƽ��������Ϊ0.64��
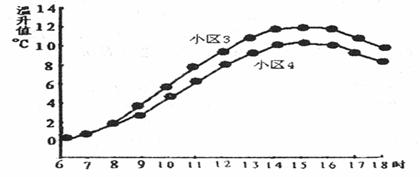
��������ͼ21��С����Ϊ���ٽϴ��С��������Ϊ3m/s��С����Ϊ���ٽ�С��С��������Ϊ2.5m/s��
 (1)���ݲ���һ�����������µ���������ʶ�С��������Ӱ�켰ԭ��4�֣�
(1)���ݲ���һ�����������µ���������ʶ�С��������Ӱ�켰ԭ��4�֣�
(2)���ݲ����������������ȵ�ǿ�ȵĹ�ϵ��ԭ��3�֣�
(3)������ʵ��С���о��ɹ���С�������滮������к���������3�֣�
(1)�µ���������С��С����������(����)С�������ʴ��С����1�֣����������µ���Դ��Ҫ�ǵ�����䣬��ͬ�µ��棬��������ͬ��1�֣���������С���µ������յ�̫�������������٣�1�֣������ͨ���������Ͷ����ͷŵ������е��������٣��������������С��1�֣���
(2)�������ȵ�ǿ��(��������)�ʸ���أ�1�֣�������Խ����������ԽС��1�֣�������Խ����������Խǿ���ɷ���ߵ�����ҲԽ�ࣨ1�֣����ɼ������ٴ������ڼ�������Ⱥ�ġ��ȵ�ЧӦ��������С���෴��
(3)�پ���ʹ�ö�̫�����������ʽ�С�Ľ������ϣ�������ö�̫�����������ʽϴ�����ࡢ����������װȫ�����棨1�֣����������̵غ�ˮ�棬�������µ���������(���ٵ��������) ��1�֣�����ͨ����������С��������(�����ܶȲ���Ҫ�ʵ�)����ǿС������Ȼͨ�磬�����ڳ���������ɢʧ���������Ӷ���Ч����С�����ȵ�ЧӦ�� ��1�֣���

���ݲ��Ϻ�ͼ���������ѧ֪ʶ���ʴ��������⡣
ij������ȤС���ij�����ĸ�סլС���Ļ������й۲����,�õ��˰��죨���죩���������ݲ�������ͼ�ס�ͼ�� (���¶�ȡij��ƽ���¶ȣ���
����һ��
�����µ����������
| �µ��� | ��·�����ࣩ | ������ | ש | ʯ | ������������ | ɳĮ | �� | ˮ |
| ������ | 0.8- 0.95 | 0.65- 0.9 | 0.6-0.8 | 0.65- 0.8 | 0.6- 0.95 | 0.55- 0.85 | 0.74- 0.84 | 0.9- 0.97 |
ע���µ��治ͬ����������Ҳ��ͬ�����ϱ��оٵ��µ����У���ˮ����������ݵش�֮��������Խ�С��
���϶���
ͼ����С��1Ϊ���ٽϴ��С����С��2Ϊ���ٽ�С��С����
 |
ͼ��
��������
ͼ����С��3Ϊ�µ��������ʽϸߵ�С����ƽ��������Ϊ0.74��С��4Ϊ�µ��������ʽϵ͵�С����ƽ��������Ϊ0.64��
 |
ͼ��
��1�����ݲ��϶������������ȵ�ǿ�ȵĹ�ϵ��ԭ��
��2�����ݲ���һ�����������µ���������ʶ�С��������Ӱ�켰ԭ��
��3����������С���������滮������ߣ���С���о��ɹ������к�������
��18�֣����ݲ��Ϻ�ͼ���������ѧ֪ʶ���ʴ��������⡣
����ʮһ���е���ʵ��С��Գ��������ĸ�סլС���Ļ������й۲����,�õ��˰���(����)6: 00��18: 00���������ݲ�������������ͼ.(���¶�ȡij��ƽ���¶�)
����һ�����µ����������
| �µ��� | �����· | ������ | ש | ʯ | �������� | ɳĮ | �� | ˮ |
| ������ | 0.9-0.95 | 0.65-0.9 | 0.6-0.8 | 0.65-0.8 | 0.6-0.95 | 0.55-0.85 | 0.74-0.84 | 0.9-0.97 |

��������С��3Ϊ���ٽϴ��С��������Ϊ3m/s;С��4Ϊ���ٽ�С��С��������Ϊ2. 5m/s.

(1)���ݲ���һ�����������µ���������ʶ�С��������Ӱ�켰ԭ��(6��)
(2)���ݲ����������������ȵ�ǿ�ȵĹ�ϵ��ԭ��(6��)
(3)������ʵ��С���о��ɹ���С�������滮������к�������(6��)
��18�֣����ݲ��Ϻ�ͼ���������ѧ֪ʶ���ʴ��������⡣
����ʮһ���е���ʵ��С��Գ��������ĸ�סլС���Ļ������й۲����,�õ��˰���(����)6: 00��18: 00���������ݲ�������������ͼ.(���¶�ȡij��ƽ���¶�)
����һ�����µ����������
|
�µ��� |
�����· |
������ |
ש |
ʯ |
�������� |
ɳĮ |
�� |
ˮ |
|
������ |
0.9-0.95 |
0.65-0.9 |
0.6-0.8 |
0.65-0.8 |
0.6-0.95 |
0.55-0.85 |
0.74-0.84 |
0.9-0.97 |
���϶���С��1Ϊ�µ��������ʽϸߵ�С����ƽ��������Ϊ0.74;С��2Ϊ�µ��������ʽϵ͵�С����ƽ��������Ϊ0.64��

��������С��3Ϊ���ٽϴ��С��������Ϊ3m/s;С��4Ϊ���ٽ�С��С��������Ϊ2. 5m/s.

(1)���ݲ���һ�����������µ���������ʶ�С��������Ӱ�켰ԭ��(6��)
(2)���ݲ����������������ȵ�ǿ�ȵĹ�ϵ��ԭ��(6��)
(3)������ʵ��С���о��ɹ���С�������滮������к�������(6��)