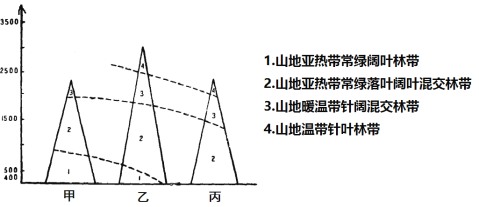
��Ŀ����
����Ŀ��25��ʱ����100 mL 0.1 mol��L1 NH4HSO4��Һ�еμ�0.1 mol��L1 NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��H2SO4��Ϊ��Ԫǿ�ᣩ������˵���������
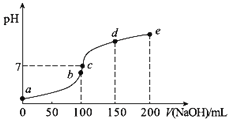
A��a��ʱ��Һ��pH<1
B��a�㵽b��Ĺ����У���Һ�ĵ�����������
C��ab���ϵĵ�(������a��)�������ϵʽ��c (![]() )��c(Na+)<2c(
)��c(Na+)<2c(![]() )
)
D��b��ʱ����Ũ�ȴ�С˳��Ϊ��c(Na+)>c(![]() )>c(
)>c(![]() )> c(H+)> c(OH)
)> c(H+)> c(OH)
���𰸡�D
��������a��Ϊ0.1 mol��L1 NH4HSO4��Һ�����������������Ũ��Ϊ0.1 mol��L1��![]() ˮ������ԣ�����a��������Ũ�ȴ���0.1 mol��L1����A��ȷ��b��������������ǡ��������H+�����ӵ����ʵ������䣬����Һ�������Ũ�ȼ�С������������������B��ȷ��ab���ϵĵ㣨������a�㣩�����ݵ���غ㣬�������ϵʽ��c (
ˮ������ԣ�����a��������Ũ�ȴ���0.1 mol��L1����A��ȷ��b��������������ǡ��������H+�����ӵ����ʵ������䣬����Һ�������Ũ�ȼ�С������������������B��ȷ��ab���ϵĵ㣨������a�㣩�����ݵ���غ㣬�������ϵʽ��c (![]() )+c(Na+)+ c(H+)=2c(
)+c(Na+)+ c(H+)=2c(![]() )+ c(OH)��ab���ϵĵ������ԣ�c(H+)> c(OH)������c(
)+ c(OH)��ab���ϵĵ������ԣ�c(H+)> c(OH)������c(![]() )+c(Na+)<2c(
)+c(Na+)<2c(![]() )����C��ȷ��b��ǡ��������H+����Һ��ֻ�е����ʵ�����(NH4)2SO4��Na2SO4��
)����C��ȷ��b��ǡ��������H+����Һ��ֻ�е����ʵ�����(NH4)2SO4��Na2SO4��![]() ˮ�⣬c(Na+)=c(
ˮ�⣬c(Na+)=c(![]() )>c(
)>c(![]() )>c(H+)> c(OH)����D����
)>c(H+)> c(OH)����D����

��ϰ��ϵ�д�
�����Ŀ