��Ŀ����
����Ŀ���Ķ����ϣ�����������⡣
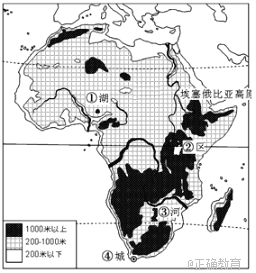
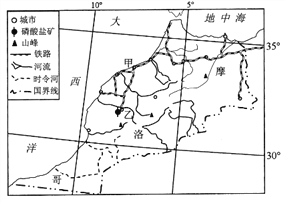
��³�˺������غ�������ͼ��λ�ڲ��ľ��ض�����������һ��һС��һ��һ����һ��ˮ��һɫ��һ������ḻ��ͼ��B�������³�˺���A���������غ���B�ӳ�����Ŵ�����ţ���������л���ע����ڣ�ʹ�������ʷʺ��Ӳݴ����������Ǹ������K��ḻ�����غ���Χȫ��ãã�ĸ��̲�����ĵ����Լ1ƽ��ǧ�ף�ÿ���������Ÿ����ͷŸ��ҰѼ�ȴ��������غ��еĵ���Ⱥ�ۣ����ϵ������ǧ�������������ѳ���ء������ۣ���ֱ�ǡ�����������������硱��
��1���ж����������ĺ�ˮ�̵����ʣ����������γ�ԭ��
��2��ָ��������Դ�ḻ�ĺ���������������ҵ��Դ�ḻ��ԭ��
��3������ÿ�������ʼ�����غ��ĺ��ĵ���Ϊ�������������Ҫԭ��
���𰸡���1����³�˺�Ϊ��ˮ�������غ�Ϊ��ˮ������³�˺��� B �����룬����A�������������������ʱ�A�Ӵ�����ʹ�η��ں��в���������³�˺���Ϊ��ˮ�������غ�ֻ��A�����룬û�к����������ηֲ��ϻ��ۣ���Ϊ��ˮ����
��2����³�˺����ú��ζȽϵͣ��ʺ��������棻�ú���������ḻ��������ϳ��㣬������ҵ��Դ�ḻ��
��3�������������������ĵ������ºͣ����ĵ��ܱ߶�dz̲�����ʳ����Դ�ḻ��������Ӱ���С�������ľ���
��������
�������Բ��ľ��ض�������������Ϊ��������������С�⣬�漰��ˮ���ʷ�����Ӱ����ҵ��Դ�����ط�������������Է��������֪ʶ�ͷ���������ѧ���Ե�����Ϣ�Ĵ�������������ѧ���ۺϷ������������������
�ڣ�1���⣬������ˮ������Ҫȡ���ں�ˮ����й��ʽ����ˮ���η���Ҫ�ɾ���ע������������ˮ��й��Ҫ������Ϊ�����ηݾͻ���ۣ��ζ����ߣ��γ���ˮ���������ˮͨ��������й��������Ѿ���ע����ηݴ������ηݲ����ں��ڻ��ۣ��γɵ�ˮ������ͼ���Կ�������³�˺���B��ע�룬A�������������γɵ�ˮ���������غ�ֻ��A��ע�룬û���������������������γ���ˮ����
�ڣ�2���⣬Ӱ����ҵ��Դ����Ҫ���ض��Ϻ�ˮ�ʣ��Ӳ����п�֪��B�ӳ�����Ŵ�����ţ���������л���ע���³�˺���Ӫ��Ԫ�طḻ���������ﷱʢ��Ϊ�������ḻ�Ķ��ϣ�ͬʱ��ǰһ���жϿ�֪���ú�Ϊ��ˮ�����ʺ϶���������������˸ú���ҵ��Դ�ḻ��
�ڣ�3���⣬��Ⱥ��Ǩ��;�У�������ͣ������Ȼ�������ơ�ʳ����Դ�ḻ�����������Ž�С��������һ˼·�ٽ��������Ϣ�����������ɵó��𰸡�