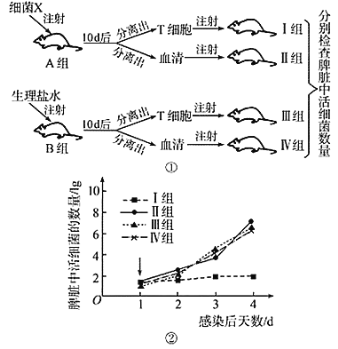
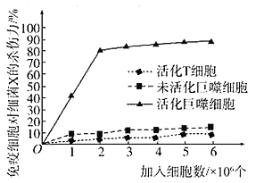
��Ŀ����
����Ŀ����ͼ��ʾ��������������Ũ�������õĹ�ϵ����ͼ��ʾ��һ����������ˮƽ���ã�����һ��ʱ���������������ͼ��ʾ������ѿ�ʡ�
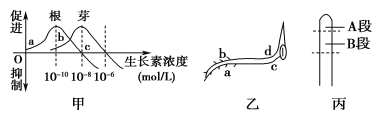
��1����ͼ�У�����ѿ��������������Ũ�ȷֱ�Ϊ________mol/L��________mol/L��c��������Ũ�ȶԸ�������ЧӦ�� ����ѿ������ЧӦ��______________��c��������Ũ�ȶ���ѿ��˵��________����ߡ��͡���Ũ�ȡ�
��2����ͼ��b��������Ũ��_________������ڡ�����С�ڡ����ڡ���a�࣬��������___________����ģ�a�������ضԾ�������ЧӦ��__________��
��3��Ϊ��֤�ڵ���������£���ͼ������ѿ�ʼ�˲����������صĺ������䷢����A�ζ����Ƿ�����B�Ρ�ijͬѧ���������ʵ�鲽�裬���������������й�ʵ����̣�
��ʵ����ϼ��þߣ�������ѿ�ʣ�һ���Ӳֽ�У�����ĸƬ����Դ�ȡ�
��ʵ����̣������Ҳ������䣬������ͼ�л��������ĸƬ��λ�ã���������������˵����
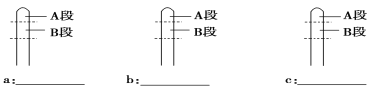
��ʵ������
a��___________________��
b��___________________��
c��___________________��
��ʵ����ۣ�_________________________________________��
���𰸡���1��10��10 10��8 �Ȳ��ٽ�Ҳ������ �ٽ� ��
��2��С�� ���� �ٽ�
��3����
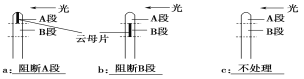
��a��ֱ������ b�������Դ���� c�������Դ����
����ѿ�ʼ�˲����������صĺ������䷢����A�ζ�����B��
����������1����ͼ������֪������ѿ��������������Ũ�ȷֱ�Ϊ10-10mol/L��10-8mol/L��c��������Ũ�ȶԸ�������ЧӦ�ǼȲ��ٽ�Ҳ�����ƣ���ѿ��ЧӦ�Ǵٽ���c��������Ũ�ȶ���ѿ��˵�ǵ�Ũ�ȡ�
��2�������ܵ��������ã���ͼ��b��������Ũ��С��a�࣬a�������ضԾ�������ЧӦ�Ǵٽ���
��3������ʵ���Ŀ������֤��������ѿ�ʼ�˲����������صĺ������䷢����A�ζ����Ƿ�����B�Ρ����ɶ�������ѿ�ʽ������ִ��������A�Σ����B�Σ�������κβ�λ�������ա������������غ�������������ѿ�ʼ�ˣ��������A�κ������ز���������䣬�����ѿ��ֱ�������������B�κ���������������䣬������ѿ�������Դ�����������������Ķ������������Դ����������������ʵ�������������Եó����ۣ���ѿ�ʼ�˲����������صĺ������䷢����A�ζ�����B�Ρ�

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�