��Ŀ����
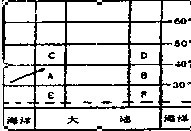
����ŷ��½�������ͷֲ�ģʽͼ�������ij������ѹ�����λ��ʾ��ͼ���ش𣺣�13�֣�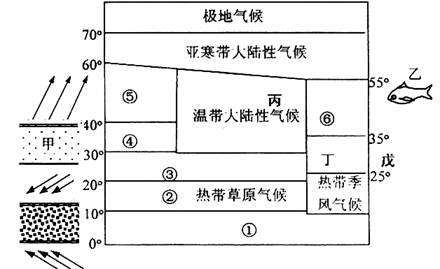
��1������ͼ�м���ѹ����λ�ã����жϱ�ͼ��ʾ�DZ������ �����ڣ���
��2����ͼ�������������͢ݷֲ���һ������� ��
�������͢۵ij����� ��
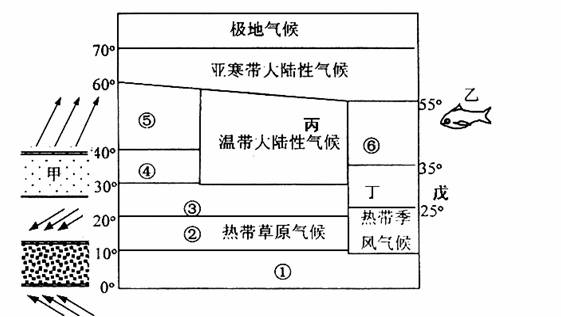
��3���������͢����ڵ���ֲ�������� ��������١��ڡ��ۡ��ܡ��ݱ仯����������Ȼ���仯��ӳ�� �ĵ��������ɡ�
��4��ͼ�ж�����λ�ڽ������꣬�õط�չũҵ�������������������� ��
ͼʾ�����������������ֺ��� ���õ�ũҵ��������ģʽ�� ��
��5���Ҹ����� �������������γɵı������泡���촦���ҹ������泡��Ϊ�ٽ�����ҵ��Դ�ɳ�����չ������������������顣
��6���������ɹ��������������죬���ҹ����۷ɴ�������½�����Է����õص�������λ������
��1�� �ļ� ��1�֣�
��2���ϱ�γ40�㡫60���½���� ��1�֣��������ܸ��ȴ���ѹ���ƣ�����ŷ������1�֣�
��3�����ȴ�����ӲҶ�֣�1�֣� �ɳ�������� ��1�֣�
��4���ļ����¶��������ͬ�� ��1�֣� ������̨�硢���꣨1�֣� ����ũҵ��1�֣�
��5��ǧ���������ձ�ů�� ��1�֣� �ʵ����̣�ʵ�������ڣ���չ��ˮ��ֳ��������ϣ�
��ǿ�������������ٺ�ˮ��Ⱦ�����躣�õĹ�������֧�֡���2�֣�
��6���ع���ϡ������ƽ̹�����ڷɴ���ȫ��½����Ӻ�������Ұ����������Ѱ����2�֣�
���������������1��ͼʾ��Ϊ30�㸽���ĸ��ȴ���ѹ������ǰ������ĸ��ȴ���ѹ����ȫλ��30��N�Ա�������˵��ȫ����ѹ��������ƣ��ʴ�ʱΪ�������ļ�����2���������͵ķֲ�����һ���γ��λ�úͺ�½λ�ù��ɣ���ֱ�Ӷ�ͼ���ɼ��ɡ�ͼʾ��λ��20��--30���½���������ж�Ϊ�ȴ�ɳĮ�����������Ҫ����ѹ�������Ӱ��Ƕȷ�������3����λ��30��--40���½�������ж�Ϊ���к�������Ȼ��Ϊ���ȴ�������Ҷ�ִ�������١��ڡ��ۡ��ܡ��ݱ仯�����ϱ��������Ȼ�����죬Ϊγ�ȵش��Թ��ɡ���4��������λ��25��--35��Ĵ�½������Ϊ���ȴ���������Ӱ�죬�ļ����¶�������ũҵ�����������ڼ�������ˮ�������ļ������ļ������γɺ����ֺ�����λ�ڶ����غ����ļ�������̨��Σ�������ڽ��������������������ϴ�Ӧ������õ��صĵ�����������չ����ũҵ��ũ���������ۺϷ�չ����5���������泡Ϊ���ձ�ů����ǧ�������������γɡ�����ҹ��غ���ҵ��չ�еĹ��Ȳ��̺ͺ�������Ӧʵʩ�������̡������ƶȵ���ҵ��Դ�Ŀɳ�����չ��ʩ������Ϻ�����̬�����ı�������ǿ���������������Ⱦ�ŷŵȷ����������6��Ӱ�캽����½������λ������Ҫ���˿ڷֲ������Ρ���Ұ�ȷ��������
���㣺���⿼��ȫ����ѹ������ķֲ��Ͷ�����ֲ���Ӱ������ݡ�
�����������Ѷ�һ�㣬������Ȼ���������ĵ������ۺϷ���������࣬�ۺ���ǿ��������Ҫ�������¼��㣺������ȫ����ѹ������ķֲ��������ɣ�������ȫ���������͵ij��ֲ������������Ȼ������ݣ��۽���ҹ�����������ſ����ж��������Ȼ�ֺ���ũҵ�����ĺ����������ܽ���泡�ij���ͺ�����Դ�ĺ��������뱣����ʩ������