��Ŀ����
��20�֣��Ķ�������ʡ������ͼ���Ͳ��ϣ���������յĵ���֪ʶ������������⡣
����һ ������ʮ�߽�����ȫ����������µ���Դ��չ�ƻ����ټ��Ϸ��ɱ��ij������ͣ��ѽӽ�ˮ�磬��Ϊ�ҹ���緢չ����������������ʡ������Դ�ܴ���Ϊ2.37��ǧ�ߣ���Ҫ�����ں������Ⱥ�ʡ�ڲ���ɽ�ڵ�����2006����ҷ���ί��ʽ���˸����Ȫǧ��ǧ���ķ����أ����ķ��������װ���Դﵽ������Ͽ�Ĺ�ģ������Ȫ����λ�ڸ������ĩ�ˣ�Զ�븺�����ģ��������ܱ�������˷�����Դ��������Ҫ��Լ���������е��������������㡣
���϶� �й��ƻ����ڽ��ĸ�����·�����쵽ŷ�ޡ����ݼƻ���������·�������ࡢ�½�ά��������������������ɼ�����˹˹̹��������˹̹�����ʵȹ�������ŷ���죬ֱ��Ӣ�����ӹŴ���˿��֮·���ڶ���ŷ��½�ţ��ٵ�ֱͨ��ŷ�ĸ�����·������ʡһֱ���ҹ�������ϵ����Ҫ��ͨҪ����
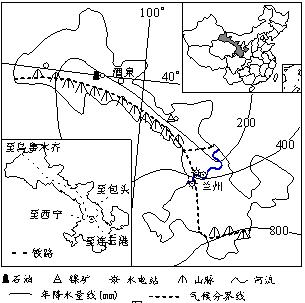
������ ����ʡ������ͼ

��1�����ݲ��Ϻ���ѧ֪ʶ����������ʡ��Ȫ�������������ص���λ���أ�6�֣�
��2���ÿɳ�����չ�Ĺ۵㣬��Ҫ��������ʡӦ������õ�����Դ����Դ�����ƹ�ҵ�ķ�չ����4�֣�
��3�������Ȫ���ҹ�Ψһ�ܹ��������˵ķ������ģ����ҹ�����Ҫ�ĺ��췢������֮һ���Լ�������Ϊ���췢�����ĵ���λ���ơ���4�֣�
��4����˵���������ʡ��������ϵ�е���λ�õ���Ҫ�ԡ���6�֣�
��1����6�֣����������ܣ�������Դ�ḻ����1�֣������Բɷֵ㣩
��ѧ�����ķ�չ���ɱ����ͣ� ��ͨ���������ڽ��裻 �ع���ϡ���Ҳ�ռ�ø��ء�����2�㣬2�֣�
�������������ܱ��������������������㣻��1�֣������Բɷֵ㣩
�ļ�������Խ�С�� ���ܱ�����ѩ�֡�ɳ�����������ֺ���Ӱ�죻 Զ�������г����������Զ������2�㣬2�֣�
��2����4�֣����÷ḻ����Դ����ɫ�������(����Ϊ��)��չ��ɫ������ҵ��ұ��ҵ������1�֣� �����ûƺ����ε�ˮ�ܡ�ʯ���Լ����ܡ�̫���ܵ���Դ��ҵ�͵�����ҵ����1�֣� ͬʱ���ɷ�չʯ��ҵ����1�֣� ���ڸ���ʡ��̬������������չ��ҵ�Ĺ����з��ι�ҵ��Ⱦ����̬�����ƻ�������1�֣�
��3����4�֣�����Ϊ��½����������࣬�����٣���2�֣������Բɷֵ㣩
����ƽ̹�������˿�ϡ�٣���Ұ������ ���ؽ���ʱ��ϳ���������ʩ���ơ�����1�㣬2�֣�
��4����6�֣���������ɳĮ�붫�ϲ�����ظ�ԭ�谭�˶�������ϵ��ʹ�����Ϊͨ��������������ϵ�Ľ�ͨҪ������2�֣� λ�ڼ�����Ҫ��·�ߣ���λ����ء����¡�¤���������ߣ��Ľ���㣬���ҹ�ͨ���½���������ص�������Ҫͨ������ͨ��Ŧ������2�֣�
���ҹ�ͨ������ϵ�����ǡ�ŷ����Ҫ��·��ͨ������2�֣�
����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�