��Ŀ����
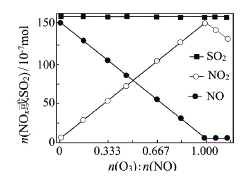
����Ŀ����t��ʱ��AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��10-10������˵������ȷ���ǣ� ��

A����t��ʱ��AgBr��KspΪ4.9��l0-13
B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b��
C��ͼ��a���Ӧ����AgBr�IJ�������Һ
D����t��ʱ��AgCl(s)+Br-(aq) ![]() AgBr(s)+Cl- (aq)��ƽ�ⳣ��K��816
AgBr(s)+Cl- (aq)��ƽ�ⳣ��K��816
���𰸡�B
��������A�������ܶȻ��Ķ��壬Ksp=c(Ag��)��c(Br��)=7��10��7��7��10��7=4.9��10��13����˵����ȷ��B��AgBr(s) ![]() Ag��(aq)��Br��(aq)�������廯�ƹ��壬c(Br��)����ʹƽ�����淴Ӧ������У�c(Ag��)��С����˵������C����ʱ��Ũ����<Ksp��˵������Һ���ڲ�������Һ����˵����ȷ��D��K=c(Cl��)/c(Br��)=c(Ag��)��c(Cl��)/[c(Ag��)��c(Br��)]=Ksp(AgCl)/Ksp(AgBr)=4��10��10/4.9��10��13��816����˵����ȷ��
Ag��(aq)��Br��(aq)�������廯�ƹ��壬c(Br��)����ʹƽ�����淴Ӧ������У�c(Ag��)��С����˵������C����ʱ��Ũ����<Ksp��˵������Һ���ڲ�������Һ����˵����ȷ��D��K=c(Cl��)/c(Br��)=c(Ag��)��c(Cl��)/[c(Ag��)��c(Br��)]=Ksp(AgCl)/Ksp(AgBr)=4��10��10/4.9��10��13��816����˵����ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ