��Ŀ����
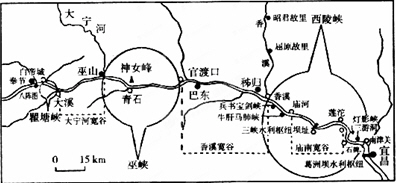
����������Ͽ�����ĵ��ʹ���ͼ�����ش�18��20�⡣

18.ͼ�Т���ʾ�ĵ��ʹ��켰Ͽ�ȣ�����ȣ��� ������
A.��б����Ͽ
B.��б�������ӿ���
C.�ϲ㡢��Ϫ����
D.���塢����Ͽ
19.�Ըõ���������������ȷ�����ǣ���
A.�������ȴ�ʪ��Ͱ�ʪ�����
B.ɽ´�ش��ֲ��д�Ƭ�ĸ�����
C.������̬��Ϊ��״
D.ɽ����Ϣ�д����Ĵ���è
20.��Ͽ��ӽ���ͼ�еģ���
A.�ٴ� B.�ڴ�
C.�۴� D.�ܴ�
18.D 19.B 20.D
����:
ͼ�Тٴ�Ϊ����Ͽ���ڴ�Ϊ��Ͽ����Ϊ��Ϫ���ȣ���Ϊ����Ͽ���ӵ��ʹ����Ͽ�������Ͽ�Ⱦ�Ϊ��б����Ͽ��ӽ��ڢܴ���

��ϰ��ϵ�д�
 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ