��Ŀ����
����Ŀ����������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��![]() ��Mg2+��Al3+��
��Mg2+��Al3+��![]() ��
��![]() ��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������õ�������Һ����Ʋ���������µ�ʵ�飺
��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������õ�������Һ����Ʋ���������µ�ʵ�飺
��֪��3![]() + 8Al + 5OH��+ 2H2O
+ 8Al + 5OH��+ 2H2O![]() 3NH3 + 8
3NH3 + 8![]()
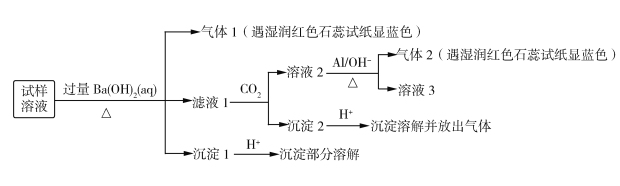
�������ϵ�ʵ������������ش�����������
��1��������Һ�п϶����ڵ�������______________________��
��2��������Һ�п��ܴ��ڵ�������__________________���жϿ��ܴ��ڵ������ӵ�ʵ�鷽��Ϊ_________________________________��
��3������1�ijɷ�Ϊ_____________��
��4��������Ԥ������������Һ���е����ʿ�����_____________���û�ѧʽ��ʾ��д���ּ��ɣ���
��5�����������Ʊ�������ƫ�����ƣ��䷴Ӧ�����ӷ���ʽΪ______________��
���𰸡���16����
��1��![]() ��Mg2+��
��Mg2+��![]() ��
��![]() ��2����
��2����
��2��Na+��Al3+��Cl ��3�֣� ȡԤ������������Һ���Թ��У���������Ba(NO3)2��Һ�����ã����ϲ���Һ�м����������ữ��AgNO3��Һ�����а�ɫ����������֤��������Һ������Cl��3�֣�
��3��BaSO4��Mg(OH)2��2�֣�
��4��NaNO3��NH4Cl������������Ҳ���ԣ���2�֣�
��5��Al3+ + 3NH3��H2O![]() Al(OH)3�� + 3
Al(OH)3�� + 3![]() ��Al(OH)3 + OH
��Al(OH)3 + OH![]()
![]() +2H2O��4�֣�
+2H2O��4�֣�
������������1������2�ǰ���������Һ��һ������![]() ������������1���Ჿ���ܽ����ɷ���������þ�����ᱵ����һ������Mg2+��
������������1���Ჿ���ܽ����ɷ���������þ�����ᱵ����һ������Mg2+��![]() ����3
����3![]() +8Al+5OH��+2H2O
+8Al+5OH��+2H2O![]() 3NH3+8
3NH3+8![]() ��֪����Һ2�к���
��֪����Һ2�к���![]() ������2�����ܽⲢ�ų����壬����2��̼�ᱵ��������Һ�����Al3+���������Ba(OH)2��Һ������Һ1����
������2�����ܽⲢ�ų����壬����2��̼�ᱵ��������Һ�����Al3+���������Ba(OH)2��Һ������Һ1����![]() ��ͨ��CO2������2һ������BaCO3��������Al(OH)3���ټ��ᣬ���������������ܽⲢ�ų����������������п��ܺ�Al3+��
��ͨ��CO2������2һ������BaCO3��������Al(OH)3���ټ��ᣬ���������������ܽⲢ�ų����������������п��ܺ�Al3+��
��1��������������˼·��֪һ�����е�������![]() ��Mg2+��
��Mg2+��![]() ��
��![]() ��
��
��2��ͨ�����Ϸ���֪�������п��ܴ���Na+��Al3+��Cl��Ҫ����������Cl�����ų�![]() �ĸ��ţ�Ȼ�����������ữ��AgNO3��Һ���顣
�ĸ��ţ�Ȼ�����������ữ��AgNO3��Һ���顣
��3������1�����Ჿ���ܽ����ɷ���BaSO4��Mg(OH)2��
��4��һ������![]() ��Mg2+��
��Mg2+��![]() ��
��![]() �����ܴ���Na+��Al3+��Cl����������������Ͽɵõ�һ�ֿ��ܵ����ʣ���NaNO3��NH4Cl��MgSO4��NaCl��Mg(NO3)2��(NH4)2SO4�ȡ�
�����ܴ���Na+��Al3+��Cl����������������Ͽɵõ�һ�ֿ��ܵ����ʣ���NaNO3��NH4Cl��MgSO4��NaCl��Mg(NO3)2��(NH4)2SO4�ȡ�
��5��Al2(SO4)3�Ʊ�������NaAlO2������Ӧ�ü��������İ�ˮ��Al3+��NH3��H2O��Ӧ����Al(OH)3���������˵õ�Al(OH)3���ټ�������NaOH��Һ�õ�NaAlO2���䷴Ӧ�����ӷ���ʽΪ��Al3++3NH3��H2O![]() Al(OH)3�� + 3
Al(OH)3�� + 3![]() ��Al(OH)3 + OH
��Al(OH)3 + OH![]()
![]() + 2H2O��
+ 2H2O��