��Ŀ����
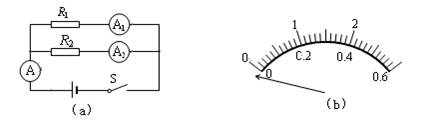
�ڡ�̽��������·���ص㡱ʵ���У�ijС��ͬѧ�õ�Դ�������������֪��ֵ�ĵ��衢������ѹ���͵����������ɸ����߽���ʵ�顣��ͨ��ʵ��õ���������·��֧·���˵�ѹ��ȡ��Ĺ��ɣ�Ȼ��ͼ��a����ʾ�ĵ�·ͼ���ӵ�·����ʵ�顣�ԽӴ����ֱ�A�ı�����ͼ��b����ʾ��������һ�����ԭ���� �����������½���ʵ�飬����ʵ�����ݼ�¼���±��С�
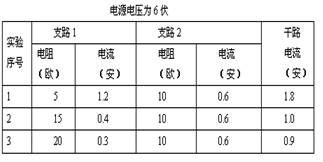
��1�������Ƚ�ʵ����� �и�·�е�����֧·1��֧·2�е�����������ϵ���ɵó��ij��������ǣ��ڲ�����·��,��·�������ڸ�֧·����֮�͡�
��2�������Ƚ�ʵ�����1��2��3�е�֧·1��֧·2������ֵ������������ɵó��ij��������ǣ���֧·2�еĵ���һ��ʱ��������·���ܵ������� ��
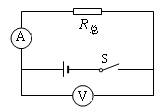
��3��Ϊ���о�������·�ܵ��������Ĺ�ϵ������ͬѧ������ԭ���ĵ�Դ(��ѹ��Ϊ6��)��������ͼ14��ʾ�ĵ�·�����õ���R���滻����ʵ���е�R1��R2������ʵ�����2��Ӧ������ֵΪ ŷ�ĵ�����ΪR����ÿ��ʵ��ʱ���۾���ҪӦ�ù۲��·�еĵ�����ʾ��,ʹ����ԭ��������· ��ʾ����ͬ��
��4����һ�������Ƚϱ��е�֧·1����I1�͵���R1�ij˻���֧·2����I2�͵���R2�ij˻��Ĺ�ϵ���ɵõ��Ľ����ǣ� ��


��1�������Ƚ�ʵ����� �и�·�е�����֧·1��֧·2�е�����������ϵ���ɵó��ij��������ǣ��ڲ�����·��,��·�������ڸ�֧·����֮�͡�
��2�������Ƚ�ʵ�����1��2��3�е�֧·1��֧·2������ֵ������������ɵó��ij��������ǣ���֧·2�еĵ���һ��ʱ��������·���ܵ������� ��
��3��Ϊ���о�������·�ܵ��������Ĺ�ϵ������ͬѧ������ԭ���ĵ�Դ(��ѹ��Ϊ6��)��������ͼ14��ʾ�ĵ�·�����õ���R���滻����ʵ���е�R1��R2������ʵ�����2��Ӧ������ֵΪ ŷ�ĵ�����ΪR����ÿ��ʵ��ʱ���۾���ҪӦ�ù۲��·�еĵ�����ʾ��,ʹ����ԭ��������· ��ʾ����ͬ��

��4����һ�������Ƚϱ��е�֧·1����I1�͵���R1�ij˻���֧·2����I2�͵���R2�ij˻��Ĺ�ϵ���ɵõ��Ľ����ǣ� ��
�����������������ӷ��ˣ���1��1��2��3����2��֧·1��������������3��6����·����������4���ڲ�����·�У���֧·�ĵ�����֧·�ĵ����С�ɷ��ȣ�
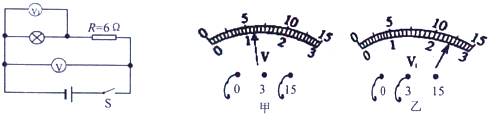
�����������ͼ��b����ʾ��������֪��������ָ�뷴��ƫת���������ڵ����������������ӷ���ɵģ�
��1���ɱ���ʵ�����Ϊ1��2��3��ʵ�����ݿ�֪��I=I1+I2���ɴ˿�֪���ڲ�����·�У���·�������ڸ�֧·����֮�ͣ�
��2���ɱ���ʵ�����1��2��3�е�֧·1��֧·2������ֵ������������ɵó��ij��������ǣ���֧·2�еĵ���һ��ʱ��������·���ܵ������� ֧·1��������������
��3����Դ��ѹΪ6V����I=U/R����ʵ�����2�е�·�ܵ���R��=U/I=6V/1A=6������ʵ�����2��Ӧ������ֵΪ6ŷ�ĵ�����ΪR����ÿ��ʵ��ʱ���۾���ҪӦ�ù۲��·�еĵ�����ʾ����ʹ����ԭ��������·��·������ʾ����ͬ��
��4���ɱ���ʵ�����ݿ�֪���ڵ�ѹһ��ʱ��֧·1����I1�͵���R1�ij˻��Ƕ�ֵ��֧·2����I2�͵���R2�ij˻����Ƕ�ֵ������֧·���������ij˻���ȣ��ɴ˿�֪���ڲ�����·�У���֧·�ĵ�����֧·�ĵ����С�ɷ��ȣ�

��ϰ��ϵ�д�
�����Ŀ