��Ŀ����
Ϊ�ˡ�̽�������ϵĵ��������˵�ѹ�Ĺ�ϵ����С�����������ͼ��ʵ���·��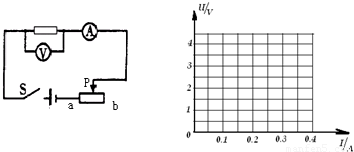
��1��ʵ��ѡ�õĵ�ԴΪ2�ڸɵ�أ���ֵ����Ϊ10�����������������С�10��2A����������ʵ��ʱ��ѹ��Ӧѡ�õ�����Ϊ______V��������Ӧѡ�õ�����Ϊ______A��
��2�������ӵ�·�Ĺ����У�����Ӧ��______�ģ������������Ļ�ƬӦ����______�ˣ���ѡ�a����b����
��3���±���С��̽���������ѹ��ϵʱ��¼�ļ������ݣ�ͨ���Ա������ݵķ������ɵó��ij��������ǣ�______��
��4������ݱ����������ݻ������õ����U-I��ϵͼ��
| ʵ����� | 1 | 2 | 3 | 4 |
| ��ѹ/V | 1.0 | 1.5 | 2.0 | 2.5 |
| ����/A | 0.10 | 0.15 | 0.20 | 0.25 |
���𰸡���������1���ɵ�Դ��ѹȷ����ѹ�����̵�ѡ���ɵ�Դ��ѹ�Ͷ�ֵ����ֵ����ȷ�����������̣�
��2�����ӵ�·�����У����ش��ڶϿ�״̬��������·���ã������������Ļ�Ƭ���������ֵ����������·���ã�
��3�������еĵ������������͵������˵ĵ�ѹ�йأ��ڵ���һ��ʱ�������еĵ������������˵ĵ�ѹ�����ȣ��ڵ������˵ĵ�ѹһ��ʱ�������еĵ���������ĵ���ɷ��ȣ�
��4�����ݱ����������������͵�ѹ�Ķ�Ӧ�㣬�ù⻬��������������
����⣺��1��ʵ��ѡ�õĵ�ԴΪ2�ڸɵ�أ����Ե�Դ��ѹ��3V�����Ե�ѹ��ѡ��0��3V���̣�
��Դ��ѹ��3V����·��С������10�������Ե�·�������ǣ�I=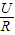 =
= =0.3A�����Ե�����ѡ��0��0.6A���̣�
=0.3A�����Ե�����ѡ��0��0.6A���̣�
��2�������ӵ�·�Ĺ����У�����Ӧ�ǶϿ��ģ���ֹ���������һ�����ߵ�·��ͨ��
�����������Ļ�Ƭ����b�������ֵ����������·�����ã�
��3���ɱ�������֪���ڵ���һ��ʱ�������еĵ������������˵ĵ�ѹ�����ȣ�
��4����ͼ�������1.0��0.10����1.5��0.15����2.0��0.20����2.5��0.25�����㣬�ù⻬������������������ͼ��

�ʴ�Ϊ����1��0��3��0��0.6����2���Ͽ���b����3���ڵ���һ��ʱ�������еĵ������������˵ĵ�ѹ�����ȣ���4������ͼ��
���������յ����е�����С��Ӱ�����أ����ÿ��Ʊ�����̽��������С��������֮��Ĺ�ϵ�����Ⲣ��������ѧ��㷨���������͵�ѹ��ͼ��������ѧ�Ƽ�����ϣ�
��2�����ӵ�·�����У����ش��ڶϿ�״̬��������·���ã������������Ļ�Ƭ���������ֵ����������·���ã�
��3�������еĵ������������͵������˵ĵ�ѹ�йأ��ڵ���һ��ʱ�������еĵ������������˵ĵ�ѹ�����ȣ��ڵ������˵ĵ�ѹһ��ʱ�������еĵ���������ĵ���ɷ��ȣ�
��4�����ݱ����������������͵�ѹ�Ķ�Ӧ�㣬�ù⻬��������������
����⣺��1��ʵ��ѡ�õĵ�ԴΪ2�ڸɵ�أ����Ե�Դ��ѹ��3V�����Ե�ѹ��ѡ��0��3V���̣�
��Դ��ѹ��3V����·��С������10�������Ե�·�������ǣ�I=
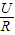 =
= =0.3A�����Ե�����ѡ��0��0.6A���̣�
=0.3A�����Ե�����ѡ��0��0.6A���̣���2�������ӵ�·�Ĺ����У�����Ӧ�ǶϿ��ģ���ֹ���������һ�����ߵ�·��ͨ��
�����������Ļ�Ƭ����b�������ֵ����������·�����ã�
��3���ɱ�������֪���ڵ���һ��ʱ�������еĵ������������˵ĵ�ѹ�����ȣ�
��4����ͼ�������1.0��0.10����1.5��0.15����2.0��0.20����2.5��0.25�����㣬�ù⻬������������������ͼ��

�ʴ�Ϊ����1��0��3��0��0.6����2���Ͽ���b����3���ڵ���һ��ʱ�������еĵ������������˵ĵ�ѹ�����ȣ���4������ͼ��
���������յ����е�����С��Ӱ�����أ����ÿ��Ʊ�����̽��������С��������֮��Ĺ�ϵ�����Ⲣ��������ѧ��㷨���������͵�ѹ��ͼ��������ѧ�Ƽ�����ϣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
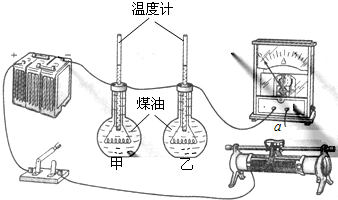 ��ѧϰ������һ�ڿ�ʱ��ͬѧ�Ǹ����ճ������������룺ͨ�絼�巢�ȶ��ٿ����������С�������С��ͨ��ʱ���йأ�����ʦ����ͬ�������ͼ��ʾ��ʵ��װ������ɲ���ʵ��̽������ƿ�еĵ���˿����ƿ�еĵ���˿�ĵ���ֵ��
��ѧϰ������һ�ڿ�ʱ��ͬѧ�Ǹ����ճ������������룺ͨ�絼�巢�ȶ��ٿ����������С�������С��ͨ��ʱ���йأ�����ʦ����ͬ�������ͼ��ʾ��ʵ��װ������ɲ���ʵ��̽������ƿ�еĵ���˿����ƿ�еĵ���˿�ĵ���ֵ��