��Ŀ����
�����������ǣ����г���̥����ѹԽ�ͣ���������Խ������������̥����ѹԽ�ͣ��ͺľ�Խ�ߣ���������ǿ�����۲췢�֣���̥����ѹ�����ǵ����������Ӵ���������һ��ԭ��������������˹���Ħ�����Ƿ�����̥�͵���Ӵ������С�йص����⣮Ϊ�ˣ����������һ��ʵ�鷽������ͬһˮƽ��·�ϣ�����������ͬһ�����г��Ͽ��Ʒ���ѣ�˫��̤�ڰ��ϲ������ֱ�������̥ѹ����£�����ǿ�õ��ɲ��������˶������������г������˶�����������ʵ�飬�õ��˲�̥ͬѹ�¹���Ħ������ƽ��ֵ���±���ʾ����̥�ŵ��������������������Բ��ƣ���
��1��������ʵ�鷽���У�������������ͬһ�����г��ϡ���Ŀ����Ϊ�˿��� �������˶������������г������˶�����Ϊ��ʹ���� ����Ħ������
��2��ͨ����ʵ�����ݵķ������Եõ��Ľ����ǣ�������������ͬ������£��Ӵ����Խ����Ħ���� ��
��3��̥ѹ���͵��������ͺ������ԭ���� ��
��1��������ʵ�鷽���У�������������ͬһ�����г��ϡ���Ŀ����Ϊ�˿��� �������˶������������г������˶�����Ϊ��ʹ���� ����Ħ������
��2��ͨ����ʵ�����ݵķ������Եõ��Ľ����ǣ�������������ͬ������£��Ӵ����Խ����Ħ���� ��
��3��̥ѹ���͵��������ͺ������ԭ���� ��

��1��ѹ�����䣻����
��2��Խ��
��3����Ϊ̥ѹ���ͻ�ʹ����Ħ��������������ͬ���ٶ���ʻ��ǣ��������������ʻ��ͬ��·�̣������������������Ĺ������࣬�������ӣ�����̥ѹ���ͻ�����ͺ�����
��2��Խ��
��3����Ϊ̥ѹ���ͻ�ʹ����Ħ��������������ͬ���ٶ���ʻ��ǣ��������������ʻ��ͬ��·�̣������������������Ĺ������࣬�������ӣ�����̥ѹ���ͻ�����ͺ�����

��ϰ��ϵ�д�
�����Ŀ
�����������ǣ����г���̥����ѹԽ�ͣ���������Խ������������̥����ѹԽ�ͣ��ͺľ�Խ�ߣ���������ǿ�����۲췢�֣���̥����ѹ�����ǵ����������Ӵ���������һ��ԭ��������������˹���Ħ�����Ƿ�����̥�͵���Ӵ������С�йص����⣮
Ϊ�ˣ����������һ��ʵ�鷽������ͬһˮƽ��·�ϣ�����������ͬһ�����г��Ͽ��Ʒ���ѣ�˫��̤�ڰ��ϲ������ֱ�������̥ѹ����£�����ǿ�õ��ɲ��������˶������������г������˶�����������ʵ�飬�õ��˲�̥ͬѹ�¹���Ħ������ƽ��ֵ���±���ʾ����̥�ŵ��������������������Բ��ƣ���
| ̥ѹ������������ | ���� | �ϵ� | �ܵ� |
| ����Ħ������ƽ��ֵ�� | 10.5N | 15.2N | 20.8N |
��2��ͨ����ʵ�����ݵķ������Եõ��Ľ����ǣ�������������ͬ������£��Ӵ����Խ����Ħ����________��
��3��̥ѹ���͵��������ͺ������ԭ����________��
 ��1��ͼ��̽���ܸ�ƽ��������ʵ��װ�ã�
��1��ͼ��̽���ܸ�ƽ��������ʵ��װ�ã�
��ʵ��ǰ�����ָܸ��Ҷ��³�����ʱ��Ӧ�Ѹܸ����˵�ƽ����ĸ��______��ѡ����ҡ������ڣ�ʹ�ܸ���______λ��ƽ�⣬��������Ŀ����Ϊ�˷���������ۣ�
���±���ijʵ��С���õ��������ݣ���������Dz����������ڶ���ʵ����������ӦΪ______ m��
| ʵ����� | ����F1/N | ������L1/m | ����F2/N | ������L2/m |
| 1 | 1.0 | 0.24 | 2.0 | 0.12 |
| 2 | 1.5 | 0.1 | 0.5 |
Ϊ�ˣ����������һ��ʵ�鷽������ͬһˮƽ��·�ϣ�����������ͬһ�����г��Ͽ��Ʒ���ѣ�˫��̤��̤���ϲ������ֱ�������̥ѹ����£�����ǿ�õ��ɲ��������˶������������г������˶�����������ʵ�飬�õ��˲�̥ͬѹ�¹���Ħ������ƽ��ֵ���±���ʾ����̥�ŵ������������������������Բ��ƣ���
| ̥ѹ | ���� | �ϵ� | �ܵ� |
| ����Ħ������ƽ��ֵ�� | 10.5N | 15.2N | 20.8N |
��ͨ����ʵ�����ݵķ������Եõ��Ľ����ǣ�������������ͬ������£��Ӵ����Խ����Ħ����______��
��̥ѹ���͵��������ͺ������ԭ���ǣ�______��
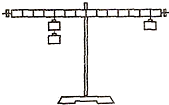 ��1��ͼ��̽���ܸ�ƽ��������ʵ��װ�ã�
��1��ͼ��̽���ܸ�ƽ��������ʵ��װ�ã�