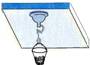
��Ŀ����
�ڲⶨ����ѹ��ʵ���У���ȱ�ٴ����̵ĵ��ɲ����ƣ�С����Ʋ����������µ�ʵ�飨��ͼ1��ʾ����
�ٽ�պˮ�����Ϲҹ����̰�ѹ�ڹ⻬ˮƽ�IJ������ϣ��������������ڵĿ�����������̵�ֱ��Ϊd��
�ڽ�װ������ϸɰ��СͰ����ع������̵����Ϲҹ��ϣ�
����С���������СͰ�ڼ�ɳ��ֱ���������̸պ����벣���壬����ƽ�����ʱСͰ��ɳ������Ϊm��
������������⣺
��1��������պˮ�����ڹ⻬ˮƽ�����ϣ���Ŀ����Ϊ��
��2����ԭ���Ͻ�����ʵ��������ѹ�ķ����Ƿ���У���
��3���������벣����ʱ�����������Ϲҹ����������������������̵�ѹ����С�ǣ�
p����ѹ=
��
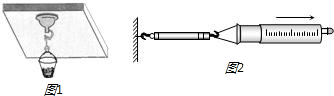
��4���������ע�������п̶Ȳ��ֵ��ݻ�ΪV�������ɲ����ơ�ϸ�ߡ��̶ȳߡ���Ƥñ����ͼ2��ʾ��ʵ��������ѹ��ֵ����д����Ҫ���裮

�ٽ�պˮ�����Ϲҹ����̰�ѹ�ڹ⻬ˮƽ�IJ������ϣ��������������ڵĿ�����������̵�ֱ��Ϊd��
�ڽ�װ������ϸɰ��СͰ����ع������̵����Ϲҹ��ϣ�
����С���������СͰ�ڼ�ɳ��ֱ���������̸պ����벣���壬����ƽ�����ʱСͰ��ɳ������Ϊm��
������������⣺
��1��������պˮ�����ڹ⻬ˮƽ�����ϣ���Ŀ����Ϊ��
�������벣����Ӵ����ܣ���ֹ������������
�������벣����Ӵ����ܣ���ֹ������������
����2����ԭ���Ͻ�����ʵ��������ѹ�ķ����Ƿ���У���
���У�������ƽ������֪ʶ
���У�������ƽ������֪ʶ
����3���������벣����ʱ�����������Ϲҹ����������������������̵�ѹ����С�ǣ�
mg
mg
����ô���ѹ��ֵ�ı���ʽ�ǣ�p����ѹ=
| 4mg |
| ��d2 |
| 4mg |
| ��d2 |
��4���������ע�������п̶Ȳ��ֵ��ݻ�ΪV�������ɲ����ơ�ϸ�ߡ��̶ȳߡ���Ƥñ����ͼ2��ʾ��ʵ��������ѹ��ֵ����д����Ҫ���裮
���������⿼���˴���ѹ�IJ�������������ƽ���������ٽ����Ŀ�ṩ���������ҳ�����ķ�����
����⣺��1��������պˮ�����ڹ⻬ˮƽ�����ϣ���Ŀ����Ϊ�˲��ÿ�����������֮�ڣ������Ӱ��ʵ������ݣ�
��2����ԭ���Ͻ�����ʵ��������ѹ�ķ����ǿ��еģ�����ȡ��ƽ�����ķ������������������µ��������ڴ����������ϵ�ѹ����Ȼ��ɼ���������ѹ����ֵ��
��3�����ݶ���ƽ���֪ʶ������ѹ��F����СͰ��ɳ������G����F=G=mg�����̵����ΪS=�У�
��2������ѹǿP=
=
��
��4��ʵ�鲽�裺
A����ע�����Ļ�������ע����Ͳ�ĵˣ�Ȼ������Ƥñ��סע������С�ף�
B����ϸ��˩סע���������ľ�����ʹ������һ���뵯�ɲ����ƵĹҹ�������Ȼ��ˮƽ������������ע����Ͳ����ע�����еĻ����ձ�����ʱ�����µ��ɲ����Ƶ�ʾ��ΪF��
C���ÿ̶ȳ߲��ע�����п̶Ȳ��ֵ��ܳ���ΪL����
D���ÿ̶ȳ߲��ע���������ƶ��ij���ΪL��
F���������ѹ��ֵ��P=
=
=
��
�ʴ�Ϊ����1���������벣����Ӵ����ܣ���ֹ�����������̣�
��2�����У�������ƽ������֪ʶ��
��3��mg��
��4��A����ע�����Ļ�������ע����Ͳ�ĵˣ�Ȼ������Ƥñ��סע������С�ף�
B����ϸ��˩סע���������ľ�����ʹ������һ���뵯�ɲ����ƵĹҹ�������Ȼ��ˮƽ������������ע����Ͳ����ע�����еĻ����ձ�����ʱ�����µ��ɲ����Ƶ�ʾ��ΪF��
C���ÿ̶ȳ߲��ע�����п̶Ȳ��ֵ��ܳ���ΪL����
D���ÿ̶ȳ߲��ע���������ƶ��ij���ΪL��
F���������ѹ��ֵ��P=
=
=
��
��2����ԭ���Ͻ�����ʵ��������ѹ�ķ����ǿ��еģ�����ȡ��ƽ�����ķ������������������µ��������ڴ����������ϵ�ѹ����Ȼ��ɼ���������ѹ����ֵ��
��3�����ݶ���ƽ���֪ʶ������ѹ��F����СͰ��ɳ������G����F=G=mg�����̵����ΪS=�У�
| d |
| 2 |
| F |
| S |
| 4mg |
| ��d2 |
��4��ʵ�鲽�裺
A����ע�����Ļ�������ע����Ͳ�ĵˣ�Ȼ������Ƥñ��סע������С�ף�
B����ϸ��˩סע���������ľ�����ʹ������һ���뵯�ɲ����ƵĹҹ�������Ȼ��ˮƽ������������ע����Ͳ����ע�����еĻ����ձ�����ʱ�����µ��ɲ����Ƶ�ʾ��ΪF��
C���ÿ̶ȳ߲��ע�����п̶Ȳ��ֵ��ܳ���ΪL����
D���ÿ̶ȳ߲��ע���������ƶ��ij���ΪL��
F���������ѹ��ֵ��P=
| F |
| S |
| F | ||
|
| L��F |
| L��V |
�ʴ�Ϊ����1���������벣����Ӵ����ܣ���ֹ�����������̣�
��2�����У�������ƽ������֪ʶ��
��3��mg��
| 4mg |
| ��d2 |
��4��A����ע�����Ļ�������ע����Ͳ�ĵˣ�Ȼ������Ƥñ��סע������С�ף�
B����ϸ��˩סע���������ľ�����ʹ������һ���뵯�ɲ����ƵĹҹ�������Ȼ��ˮƽ������������ע����Ͳ����ע�����еĻ����ձ�����ʱ�����µ��ɲ����Ƶ�ʾ��ΪF��
C���ÿ̶ȳ߲��ע�����п̶Ȳ��ֵ��ܳ���ΪL����
D���ÿ̶ȳ߲��ע���������ƶ��ij���ΪL��
F���������ѹ��ֵ��P=
| F |
| S |
| F | ||
|
| L��F |
| L��V |
����������ѹ�IJ���һ�����ƽ�ⷨ������ӻ�ô���ѹ����ֵ����Ŀ�еķ������У��������ѹ����ʵֵ����һ���IJ��죮

��ϰ��ϵ�д�
�����Ŀ
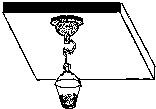
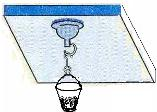
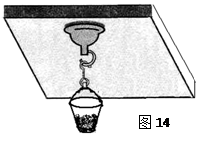 �ٽ�պˮ�����Ϲҹ����̰�ѹ�ڹ⻬ˮƽ���컨���ϣ��������������ڵĿ�����������̵�ֱ��Ϊd��
�ٽ�պˮ�����Ϲҹ����̰�ѹ�ڹ⻬ˮƽ���컨���ϣ��������������ڵĿ�����������̵�ֱ��Ϊd��