��Ŀ����
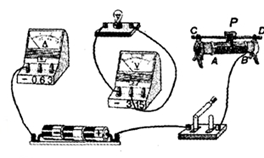
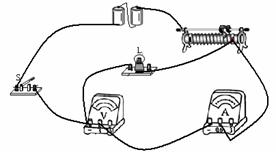
��ͼ��ʾ��С���ڽ��С�����С���ݵ繦�ʡ���ʵ�飬���������˲��ֵ�·����֪��Դ��ѹΪ3V��С���ݵĶ��ѹΪ2.5V��
��1�����ñʻ��ߴ��浼�ߣ���ͼʾ��·����������
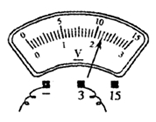
��2�����Ӻõ�·�պϿ��أ��ƶ������������Ļ�Ƭ������С����ʼ�ղ�������������ʾ������ѹ��ʾ��Ϊ�㣬����ֹ��ϵ�ԭ������� ��
��3�������ų���С��ͨ��ʵ�����������ݣ��������ݿ�֪���ݵĶ������ W��
��4��С����ͨ��ʵ�����Ƚϵ��ݡ�ʵ�ʵ�ѹ�Ƕ��ѹһ��ʱ�ĵ繦��P1���͡�ʵ�ʵ����Ƕ����һ��ʱ�ĵ繦��P2���Ĵ�С����С����ͬѧû��ͨ��ʵ��Ҳ�Ƚϳ������ǵĴ�С��P1Ӧ P2��ѡ����ڡ�����С�ڡ����ڡ�����

��1�����ñʻ��ߴ��浼�ߣ���ͼʾ��·����������
��2�����Ӻõ�·�պϿ��أ��ƶ������������Ļ�Ƭ������С����ʼ�ղ�������������ʾ������ѹ��ʾ��Ϊ�㣬����ֹ��ϵ�ԭ������� ��
��3�������ų���С��ͨ��ʵ�����������ݣ��������ݿ�֪���ݵĶ������ W��
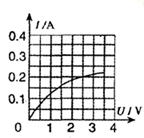
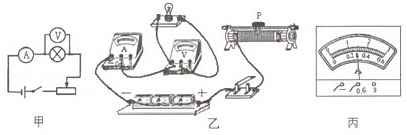
| ʵ����� | ��ѹ��ʾ��U/V | ������ʾ��I/A |
| 1 | 2.0 | 0.17 |
| 2 | 2.5 | 0.20 |
| 3 | 3.0 | 0.22 |
��1����ͼ��ʾ ��2�����ݶ�·�� ��3��0.5�� ��4������


�����������1�������������ѽ�������Ľ��������ٽ�������һ����������

��2����������ʾ����˵����·ͨ·�����ݲ�������ѹ����ʾ����˵�����ݶ�·����ѹ����ߵ�ѹ��ʾ��Ϊ0��
��3�����ݵĶ�繦��
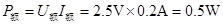 ��
����4���ɱ����м�¼�����ݿ�֪��С���ݵĵ��費�Ƕ�ֵ���������ŵ�ѹ�����������С���ݵ�ʵ�ʵ�ѹΪ���ѹ��һ��ʱ��С���ݵĵ���С����������ʱ�ĵ��裬ͨ��С���ݵĵ�������С���ݶ������һ�룬����С���ݵ�ʵ�ʵ�ѹΪ���ѹһ��ʱ�Ĺ���P1����ʵ�ʵ���Ϊ�����һ��ʱ�Ĺ���P2��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
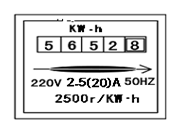
 ��5�³����ܱ���ͼ��ʾ���������ҹ�ʹ����______�ȵ磮������۲��˼����ӣ�������ܱ���ת��ת��60Ȧ�����⼸���������ҵ��õ������ĵĵ�����______J��
��5�³����ܱ���ͼ��ʾ���������ҹ�ʹ����______�ȵ磮������۲��˼����ӣ�������ܱ���ת��ת��60Ȧ�����⼸���������ҵ��õ������ĵĵ�����______J��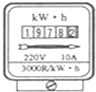
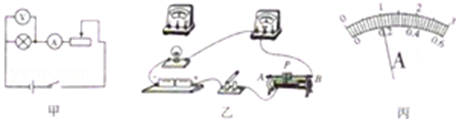

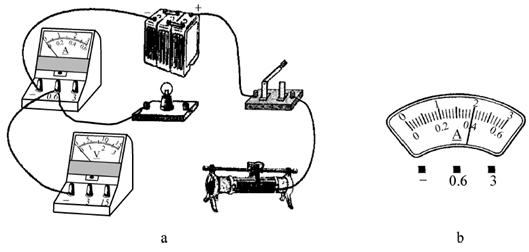
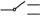 ��������ԭ�е�ʵ�����ģ�˳�������ʵ�飮��������������ʵ�飬Ҫ���·ֻ����һ�Σ������ʵ�鷽����
��������ԭ�е�ʵ�����ģ�˳�������ʵ�飮��������������ʵ�飬Ҫ���·ֻ����һ�Σ������ʵ�鷽����