��Ŀ����
22���Ķ�������ģ�
���������磬��Ե�岻�����磮��һЩ���ϣ������������ڵ���;�Ե��֮�䣬�����뵼�壬���˵��������⣬�뵼�廹����������ĵ�ѧ���ܣ�ʹ������˶�����ҪӦ�ã�
�еİ뵼�壬�����Ⱥ����Ѹ�ټ�С����֮���������¶ȵĽ��Ͷ�Ѹ�ٵ������������ְ뵼��������������С���������裮���������������������С��Χ�ڵ��¶ȱ仯����Ӧ�죬���Ҿ��ȸߣ�
�ش��������⣺

��1������������������Դ����������һ����ֵ���贮����һ����·����ͼ19����ʾ�����������ز��䣬ֻҪ������������������¶Ƚ��ͣ���·�е�������
��2��������·�У����ǽ��������еĵ����̶��̻�����Ӧ���¶ȿ̶��̣�����ֱ����ʾ���������踽�����¶�ֵ������̶������е��¶ȿ̶�ֵΪ20�棨��ͼ����ʾ������15��Ŀ̶�Ӧ��20���
��3�������������·�У�����������Ϊ��ѹ�����õ�ѹ����ʾ����ӳ�������踽�����¶�ֵ��Ҫ���ѹ���������ָ��ƫת������ͬʱ������ӳ���¶ȱ仯����Ҳ��ͬ�������ͼ���ĵ�·��ƣ�
���������磬��Ե�岻�����磮��һЩ���ϣ������������ڵ���;�Ե��֮�䣬�����뵼�壬���˵��������⣬�뵼�廹����������ĵ�ѧ���ܣ�ʹ������˶�����ҪӦ�ã�
�еİ뵼�壬�����Ⱥ����Ѹ�ټ�С����֮���������¶ȵĽ��Ͷ�Ѹ�ٵ������������ְ뵼��������������С���������裮���������������������С��Χ�ڵ��¶ȱ仯����Ӧ�죬���Ҿ��ȸߣ�
�ش��������⣺

��1������������������Դ����������һ����ֵ���贮����һ����·����ͼ19����ʾ�����������ز��䣬ֻҪ������������������¶Ƚ��ͣ���·�е�������
С
�����С��������2��������·�У����ǽ��������еĵ����̶��̻�����Ӧ���¶ȿ̶��̣�����ֱ����ʾ���������踽�����¶�ֵ������̶������е��¶ȿ̶�ֵΪ20�棨��ͼ����ʾ������15��Ŀ̶�Ӧ��20���
��
�ߣ�����ҡ�������3�������������·�У�����������Ϊ��ѹ�����õ�ѹ����ʾ����ӳ�������踽�����¶�ֵ��Ҫ���ѹ���������ָ��ƫת������ͬʱ������ӳ���¶ȱ仯����Ҳ��ͬ�������ͼ���ĵ�·��ƣ�
��������1�������������������ʱ����Ѹ�ټ�С���¶Ƚ���ʱ����Ѹ�ٵ��ص㣬���Ե�������������������¶Ƚ��ͣ���ֵ������ŷķ���ɿ�֪��·�еĵ����仯�����
��2�����������踽�����¶�С��20��ʱ�������������ֵ���·�еĵ�����С������15��Ŀ̶�Ӧ��20�����ߣ�
��3������������Ϊ��ѹ�����õ�ѹ����ʾ����ӳ�������踽�����¶�ֵ������ѹ����������������������ʱ����������������������¶Ƚ��ͣ���ֵ������������ֵĵ�ѹ����ѹ����ʾ��������ѹ����������R0����ʱ����������������������¶Ƚ��ͣ���ֵ������������ֵĵ�ѹ����R0���˵ĵ�ѹ��С���ݴ��жϵ�ѹ�����ӵ�λ�ã�������·ͼ��
��2�����������踽�����¶�С��20��ʱ�������������ֵ���·�еĵ�����С������15��Ŀ̶�Ӧ��20�����ߣ�
��3������������Ϊ��ѹ�����õ�ѹ����ʾ����ӳ�������踽�����¶�ֵ������ѹ����������������������ʱ����������������������¶Ƚ��ͣ���ֵ������������ֵĵ�ѹ����ѹ����ʾ��������ѹ����������R0����ʱ����������������������¶Ƚ��ͣ���ֵ������������ֵĵ�ѹ����R0���˵ĵ�ѹ��С���ݴ��жϵ�ѹ�����ӵ�λ�ã�������·ͼ��
���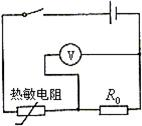 �⣺��1����������������ص��֪����������������������¶Ƚ��ͣ���ֵ������ŷķ���ɿ�֪��·�еĵ�����С��
�⣺��1����������������ص��֪����������������������¶Ƚ��ͣ���ֵ������ŷķ���ɿ�֪��·�еĵ�����С��
��2�����������踽�����¶�С��20���Ϊ15��ʱ�������������ֵ���·�еĵ�����С������15��Ŀ̶�Ӧ��20�����ߣ�
��3������������Ϊ��ѹ�����õ�ѹ����ʾ����ӳ�������踽�����¶�ֵ������ѹ����������R0����ʱ����������������������¶Ƚ��ͣ���ֵ������������ֵĵ�ѹ����R0���˵ĵ�ѹ��С����ѹ����ʾ����С������Ҫ��·ͼ��ͼ��ʾ��
�ʴ�Ϊ����1��С��
��2����
��3����ͼ��ʾ��
 �⣺��1����������������ص��֪����������������������¶Ƚ��ͣ���ֵ������ŷķ���ɿ�֪��·�еĵ�����С��
�⣺��1����������������ص��֪����������������������¶Ƚ��ͣ���ֵ������ŷķ���ɿ�֪��·�еĵ�����С����2�����������踽�����¶�С��20���Ϊ15��ʱ�������������ֵ���·�еĵ�����С������15��Ŀ̶�Ӧ��20�����ߣ�
��3������������Ϊ��ѹ�����õ�ѹ����ʾ����ӳ�������踽�����¶�ֵ������ѹ����������R0����ʱ����������������������¶Ƚ��ͣ���ֵ������������ֵĵ�ѹ����R0���˵ĵ�ѹ��С����ѹ����ʾ����С������Ҫ��·ͼ��ͼ��ʾ��
�ʴ�Ϊ����1��С��
��2����
��3����ͼ��ʾ��
����������ؼ���һ�����ú�����������ص㣬�������úô�����·�ķ�ѹ��ϵ��

��ϰ��ϵ�д�
�����Ŀ