��Ŀ����
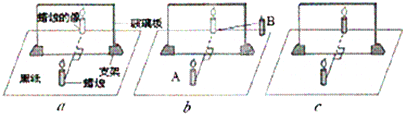
 �ڡ�̽��ƽ�澵������ص㡱ʵ���У�С���ò����塢��ͬ����������A��B���̶ȳߡ���Ƥ�ࡢ��ֽ�����Ľ���ʵ�飬��ͼ��ʾ��
�ڡ�̽��ƽ�澵������ص㡱ʵ���У�С���ò����塢��ͬ����������A��B���̶ȳߡ���Ƥ�ࡢ��ֽ�����Ľ���ʵ�飬��ͼ��ʾ����1��ʵ��ʱ������Ӧ��
��ֱ
��ֱ
������ˮƽ�����ϣ���2��ʵ������ѡ����ͬ����������A��B����Ŀ����Ϊ��
�Ƚ������Ĵ�С
�Ƚ������Ĵ�С
����3���ò��������ƽ�澵����Ҫ�����ò����������ص㣬����
ȷ�����λ��
ȷ�����λ��
����4��ʵ����ʹ�ÿ̶ȳߣ���Ϊ�˲���
�������
�������
����5��Ϊ��˵��ƽ�澵�����ɵ�����ʵ��������Ӧ��ȡ�ľ������������
�����λ���Ϸ�һ����
�����λ���Ϸ�һ����
���������ϳнӲ�����
�����ϳнӲ�����
����ƽ�澵���ɵ���Ϊ��
��
���ʵ�����顱������6��С����ʵ��ʱ���ֱ������������ȵ�һ�����ݣ��͵ó�ʵ�����֮һ��������������ȡ�������Ϊ���ַ����Ƿ������
������
������
�������ǣ�ʵ�����̫�٣����кܴ��żȻ��
ʵ�����̫�٣����кܴ��żȻ��
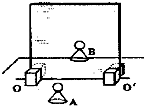
����7��С����ʵ������У��ò�������00'�ᣨ������������ĽӴ����֣�����ˮƽ�����ƶ�����������־��е��������A
��
��
�ƶ���ѡ����ҡ������������������ֽ���������00����ת���Լ������־��е���ת���Լ�
ת���Լ�
��ѡ�ת���Լ�������ת���Լ���������������������1����������ڲ�����Գƣ�������İڷŽǶ�Ҫ�ܹ�ʹ�����������ǰ�����ӵ�����ȫ�غϣ�
��2��ʵ������A������B������ȫ��ͬ��B����A������ȫ�غϣ�����͵ó�A������A��С��ͬ�Ľ��ۣ�
��3�����ò����������ص㣬���Թ۲쵽���������һ�࣬�����ҵ����λ�ã�
��4��ʵ���п̶ȳߵ���;�ǿ��Բ������������ƽ�澵�ľ��룻
��5��ƽ�澵���ɵ����Ƿ�����ߵķ����ӳ���۶��ɵģ�����ʵ�ʹ���۳ɶ������Գɵ����������ù������ղ�����ֻ�����۾��۲죻
��6�����ɵĵó���ͨ��������ʵ�����ݹ����ܽ�����ģ�ͨ��һ��ʵ�����ݾ��ܽ�����ۣ����кܴ��żȻ�ԣ�Ҫ�������ʵ�����ʹ�õ��Ĺ��ɸ����ձ��ԣ�
��7������ƽ�澵�����ص��֪��������ھ����ǶԳƵĿɽ����⣮
��2��ʵ������A������B������ȫ��ͬ��B����A������ȫ�غϣ�����͵ó�A������A��С��ͬ�Ľ��ۣ�
��3�����ò����������ص㣬���Թ۲쵽���������һ�࣬�����ҵ����λ�ã�
��4��ʵ���п̶ȳߵ���;�ǿ��Բ������������ƽ�澵�ľ��룻
��5��ƽ�澵���ɵ����Ƿ�����ߵķ����ӳ���۶��ɵģ�����ʵ�ʹ���۳ɶ������Գɵ����������ù������ղ�����ֻ�����۾��۲죻
��6�����ɵĵó���ͨ��������ʵ�����ݹ����ܽ�����ģ�ͨ��һ��ʵ�����ݾ��ܽ�����ۣ����кܴ��żȻ�ԣ�Ҫ�������ʵ�����ʹ�õ��Ĺ��ɸ����ձ��ԣ�
��7������ƽ�澵�����ص��֪��������ھ����ǶԳƵĿɽ����⣮
����⣺��1����������岻��ֱ�������������ǰ�����ӵ�����ͬһ�߶��ϣ����������������غϵģ����Ҳ�����Ƚ�������Ĵ�С��ϵ��
�ʴ�Ϊ����ֱ��
��2������ʵ��Ҫ��ѡ�������ӵĹ������ȫ��ͬ�ģ�Ŀ����ͨ���ȽϿ���������Ĵ�С��ϵ��
�ʴ�Ϊ���Ƚ������Ĵ�С��
��3�����IJ����壬���Թ۲쵽���������һ�࣮���ڹ۲쵽A�������ͬʱ��Ҳ�ܹ۲쵽����B��ȷ�����λ�úʹ�С��
�ʴ�Ϊ��ȷ�����λ�ã�
��4����ʵ�����õ��̶ȳߣ����߲����Dz�������Ĵ�С�õģ����Dz����ľ��롢�ﵽ���ľ��룬Ȼ��Ƚ϶��߹�ϵ�õģ�
�ʴ�Ϊ��������࣮
��5��Ϊ��ȷ��ƽ�澵�ɵ�����ʵ�����������������λ���Ϸ�һ�������������ϳнӲ�������ƽ�澵���ɵ���Ϊ����
�ʴ�Ϊ�������λ���Ϸ�һ�����������ϳнӲ���������
��6������һ��ʵ��͵ó����ۣ����кܴ��żȻ�ԣ�Ϊ�˱���һ��ʵ���żȻ�ԣ�Ӧ��β������ҳ��ձ���ɣ�
�ʴ�Ϊ����������ʵ�����̫�٣����кܴ��żȻ�ԣ�
��7��������������ھ����ǶԳƵģ���������OO������ת��ʱ����ͼ��ʾ��������Ҳ���˵ķ���ת����

�ʴ�Ϊ������ת���Լ���
�ʴ�Ϊ����ֱ��
��2������ʵ��Ҫ��ѡ�������ӵĹ������ȫ��ͬ�ģ�Ŀ����ͨ���ȽϿ���������Ĵ�С��ϵ��
�ʴ�Ϊ���Ƚ������Ĵ�С��
��3�����IJ����壬���Թ۲쵽���������һ�࣮���ڹ۲쵽A�������ͬʱ��Ҳ�ܹ۲쵽����B��ȷ�����λ�úʹ�С��
�ʴ�Ϊ��ȷ�����λ�ã�
��4����ʵ�����õ��̶ȳߣ����߲����Dz�������Ĵ�С�õģ����Dz����ľ��롢�ﵽ���ľ��룬Ȼ��Ƚ϶��߹�ϵ�õģ�
�ʴ�Ϊ��������࣮
��5��Ϊ��ȷ��ƽ�澵�ɵ�����ʵ�����������������λ���Ϸ�һ�������������ϳнӲ�������ƽ�澵���ɵ���Ϊ����
�ʴ�Ϊ�������λ���Ϸ�һ�����������ϳнӲ���������
��6������һ��ʵ��͵ó����ۣ����кܴ��żȻ�ԣ�Ϊ�˱���һ��ʵ���żȻ�ԣ�Ӧ��β������ҳ��ձ���ɣ�
�ʴ�Ϊ����������ʵ�����̫�٣����кܴ��żȻ�ԣ�
��7��������������ھ����ǶԳƵģ���������OO������ת��ʱ����ͼ��ʾ��������Ҳ���˵ķ���ת����

�ʴ�Ϊ������ת���Լ���
����������ƽ�澵�����ص��ʵ��̽���������п�������ȵ㣬�ܽ������¿��㣬��ͬѧ������ԵĽ��и�ϰ��
̽��ƽ�澵�����ص�ʵ��
��1��ѡ��ʲô��Ϊƽ�澵�������壮
Ϊʲôѡ���������ѡ���ӣ�Ϊ��ȷȷ�����λ�ã�
��2����ʵ���е���ֻ������ʲôҪ����ȫ��ͬ��
Ϊʲôѡ����ֻ��ȫ��ͬ������Ϊ�˱Ƚ�������Ĵ�С��
��3��ʵ���в������������ã���ֱ�����森
������û�����洹ֱ��ʵ�����ʲôӰ�죺
���������ƶ����������������ǰ�����������ȫ�غϣ�
��4����������ܷ��ù������յ������ܣ�
�����ù������յ���˵��ʲô��ƽ�澵�ɵ���������
��5��ʵ��ʱ����һֻ�ȴ��������ǰ�����������ȫ�غϣ�Ŀ���ǣ�
�ٱȽ�������Ĵ�С����ȷ�����λ�ã�
��6���̶ȳ��ڴ�ʵ���е����ã��Ƚ������ﵽ�������Ĺ�ϵ
��7��һֻ�����ڲ��������ȴ����������ԭ���ǣ������������������森
������֤���������Dz������������������ԭ����һ�������������ʵ�飮
��8����֤�����ﵽ��������ϵʱ��Ҫ���ж��ʵ�飬Ŀ���ǣ��ҵ����ձ�Ĺ��ɣ�
���ʵ��Ҫ�ı�ʲô���ı������λ�ã�
��9���������Ӵ����������ʵ��ʱ��ȱ���ǣ���Ƚϰ��������壮
��������ʵ��ʱ���������������ֵ�Ͳ����������ǰ��������ӣ�
��10��ƽ�澵�����ԭ���ǣ���ķ��䣮
��11���ڽϰ���ʵ��Ч�����ã�
̽��ƽ�澵�����ص�ʵ��
��1��ѡ��ʲô��Ϊƽ�澵�������壮
Ϊʲôѡ���������ѡ���ӣ�Ϊ��ȷȷ�����λ�ã�
��2����ʵ���е���ֻ������ʲôҪ����ȫ��ͬ��
Ϊʲôѡ����ֻ��ȫ��ͬ������Ϊ�˱Ƚ�������Ĵ�С��
��3��ʵ���в������������ã���ֱ�����森
������û�����洹ֱ��ʵ�����ʲôӰ�죺
���������ƶ����������������ǰ�����������ȫ�غϣ�
��4����������ܷ��ù������յ������ܣ�
�����ù������յ���˵��ʲô��ƽ�澵�ɵ���������
��5��ʵ��ʱ����һֻ�ȴ��������ǰ�����������ȫ�غϣ�Ŀ���ǣ�
�ٱȽ�������Ĵ�С����ȷ�����λ�ã�
��6���̶ȳ��ڴ�ʵ���е����ã��Ƚ������ﵽ�������Ĺ�ϵ
��7��һֻ�����ڲ��������ȴ����������ԭ���ǣ������������������森
������֤���������Dz������������������ԭ����һ�������������ʵ�飮
��8����֤�����ﵽ��������ϵʱ��Ҫ���ж��ʵ�飬Ŀ���ǣ��ҵ����ձ�Ĺ��ɣ�
���ʵ��Ҫ�ı�ʲô���ı������λ�ã�
��9���������Ӵ����������ʵ��ʱ��ȱ���ǣ���Ƚϰ��������壮
��������ʵ��ʱ���������������ֵ�Ͳ����������ǰ��������ӣ�
��10��ƽ�澵�����ԭ���ǣ���ķ��䣮
��11���ڽϰ���ʵ��Ч�����ã�

��ϰ��ϵ�д�
�����Ŀ
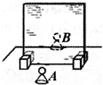 28���ڡ�̽��ƽ�澵������ص㡱ʵ���У�ijͬѧ�ò����塢��ͬ����������A��B���̶ȳߡ���ֽ����Ƥ������Ľ���ʵ�飬����ͼ��ʾ��
28���ڡ�̽��ƽ�澵������ص㡱ʵ���У�ijͬѧ�ò����塢��ͬ����������A��B���̶ȳߡ���ֽ����Ƥ������Ľ���ʵ�飬����ͼ��ʾ��